Sudden cardiac death in a dog during Holter recording-R on T phenomenon

Author information
Gunasekaran T., Sanders R.A. Sudden cardiac death in a dog during Holter recording-R on T phenomenon // J Vet Cardiol. 2017 Oct;19(5):455-461.
Abstract
A 6-year-old castrated male Golden Retriever was diagnosed with severe subaortic stenosis with severe left atrial enlargement and high heart rate due to atrial fibrillation. Treatment with digoxin and diltiazem to control ventricular response rate was initiated. Ambulatory electrocardiographic monitoring (Holter monitoring) was performed at the beginning of treatment and was repeated to evaluate the patient's response to drug therapy. Drug dose adjustments were made based on response to therapy as assessed by Holter monitoring. The dogexperienced sudden death at home 19 days after beginning treatment while wearing the Holter monitor. Analysis of the Holter recording revealed marked increase in number and complexity of ventricular arrhythmias. A ventricular premature complex occurring on a T wave (R on T) was noted preceding the polymorphic ventricular tachycardia. This arrhythmia immediately degenerated into ventricular fibrillation followed by asystole. This case report describes the arrhythmia that preceded cardiac arrest and reviews the risk factors that could have potentiated the fatal arrhythmia in this dog.
KEYWORDS: Atrial fibrillation; Canine; Subaortic stenosis; Ventricular fibrillation
Abbreviations
- AF atrial fibrillation
- ECG electrocardiogram
- HR heart rate
- LQTS long QT syndrome
- MI myocardial infarction
- PVT polymorphic ventricular tachycardia
- QTAF QT interval calculated in atrial fibrillation
- SAS subaortic stenosis
- SCD sudden cardiac death
- TdP torsade de pointes
- VA ventricular arrhythmia
- VF ventricular fibrillation
- VPC ventricular premature complex
- VT ventricular tachycardia
A 6-year-old 46.6-kg castrated male Golden Retriever dog was referred to Michigan State University Veterinary teaching hospital for evaluation of cardiomegaly. Before evaluation, the dog was reported to have been lethargic with a history of a non-productive cough. Physical examination findings included; a left basal murmur (grade III/VI) ausculted during systole on cardiac auscultation and an irregularly irregular tachyarrhythmia with pulse deficits. Respiratory rate was 24 breaths/min with normal bronchovesicular sounds on thoracic auscultation.
A 5-min in-hospital electrocardiogram (ECG) revealed absent P waves and a very high ventricular rate (approximately 240 bpm) consistent with atrial fibrillation (AF; see Fig. 1). Ventricular arrhythmias (VA) were not notedduringthe 5-min diagnostic ECG. The average corrected QT interval calculated in atrial fibrillation (QTAF method [1] described later in the discussion section) measured over 10 s on this ECG was 0.278 s. Transthoracic echocardiography was performed revealing severe dilatation of the left atrium [left atrial systolic diameter 59 mm (body weight adjusted reference range is 28—29 mm in M- mode [2])), left atrial-to-aortic ratio 2.4 (reference ratio is 0.9—1.6) [2]], and an obstructive ridge below the aortic valve consistent with subaortic stenosis (SAS).
Indices of systolic function were measured over 10 consecutive beats (fractional shortening % = 21% (M-mode reference range 39 ± 6% [3]) and ejection fraction = 42% (Normal M-mode ejection fraction is 60—80%) [4]) which were consistent with mild systolic dysfunction. The heart rate (HR) how¬ever was very high (224 bpm) during echocardiog¬raphy, which may have affected the systolic function. Spectral Doppler evaluation of transaortic velocity revealed variable waveform patterns and velocities with a peak transaortic velocity of 4.98 m/ s.
The average transaortic velocity obtained over ten consecutive beats was3.79 m/s (range 2.79—4.98 m/ s, trans-aortic velocity reference range = 1.06 ± 0.21 m/sec [5]). The variation in transaortic velocities was assumed to be associated with the arrhythmia. A diagnosis of severe SAS with rapid ventricular rate secondary to AF was made based on the echocardiographic and ECG findings. Complete blood chemistry evaluation and thoracic radiographs were recommended but were declined by the owner due to financial constraints. Treatment to control the ventricular response rate was initiated with digoxin (0.004 mg/kg, PO q12h), and diltiazem (0.645 mg/kg, PO, q8h). A Holter monitor with 7-day recording capacity was placed to assess the dog’s HR while initiating therapy at no cost to the owner as part of an ongoing research study. The dog’s owner contacted us the next day reporting improved activity level but a persisting cough. Trial therapy with furosemide (0.86 mg/kg, PO, q12h) was initiated. At the 48-h recheck appointment, the owners reported improvement in the dog’s activity level and a resolution of his cough.
A 5-min diagnostic ECG revealed persisting high rate AF (HR approximately 200 bpm).
Figure 1 Representative portion of the electrocardiogram at the time of initial evaluation from a dog diagnosed with severe subaortic stenosis.
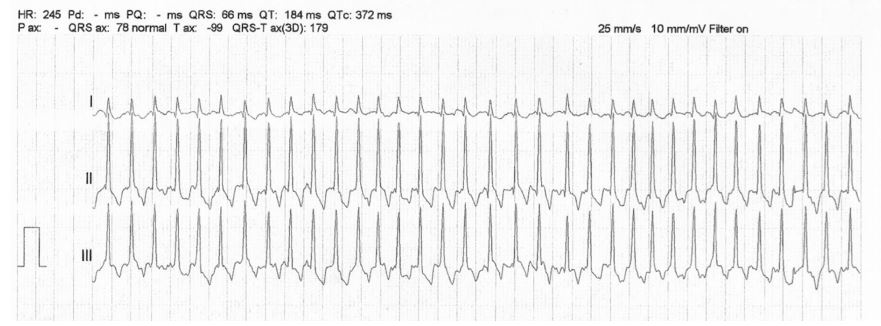
The underlying rhythm has no obvious P waves with an undulating baseline, irregularly irregular R—R intervals (mean 1-min heart rate of 240 bpm) and a narrow QRS complex consistent with atrial fibrillation. The corrected QTAF interval is 0.272 s. Paper speed 25 mm/s; 10 mm/mV. QTAF, QT interval calculated in atrial fibrillation.
The first 48 h of the Hotter recording was briefly analyzed using an ECG screening toolc by a board-certified cardiologist (RAS) at the time of recheck to determine the dog’s mean home HR. This software system does not allow for any detailed analysis of arrhythmias and as such a complete analysis was not performed at that time. Mean 48 h HR (obtained from 1-min averages) was elevated (mean HR = 185 bpm, maximum HR = 250 bpm, and minimum HR = 137 bpm). Further diagnostic tests, including a complete blood chemistry, urinalysis, and thoracic radiographs, were recommended but declined by the owner due to financial constraints. Given that the mean HR was still elevated, the dose of diltiazem was increased (0.8 mg/kg, PO, q8h). Furosemide and digoxin dosages were maintained as previously directed. The Holter monitoring was continued for 5 more days and the data were reviewed by a board-certified cardiologist (RAS) revealing a mean HR of 160 bpm (maximum HR = 251 bpm, and minimum HR = 98 bpm). At this time, the entire recording period was also com¬pletely evaluated using a Holter analysis systemd to determine the frequency and complexity of any VA. There were complex VA noted on the first 24 h of the Holter recording (Table 1). However, the frequency and complexity of the VAwere much lowerduringthe remainder of the Holter recording (Table 1). There was no variation in QTAF interval from day 1 to day 7 of the Holter recording noted (Table 1). Marked improvement in the dog’s activity level was observed during this time.
No changes in therapy were made based on clinical improvement, well-controlled HR and much improved VA status. After 12 days of therapy, a 24-h Holter monitor was repeated. Unfortunately, the dog died at home while wearing the Holter monitor. Retrospective analysis of the 24-h Holter recording before sudden cardiac death (SCD) by a board-certified cardiologist (RAS) revealed well-controlled HR, but a marked increase in number and complexity of VA com¬pared to the previously obtained Holter recordings (Table 1). Additionally, there was a mild prolongation in the average QTAF interval compared with the previous Holter recording (Table 1).
Description of the pre-arrest rhythm
The representative portion of the Holter record¬ing immediately preceding SCD is depicted in c Lifescreen, Spacelabs Heatlhcare, Snoqualmie, WA. d Pathfinder SL, Spacelabs Healthcare, Snoqualmie, WA.
Fig. 2A and B. The pair of tracings in each row represents channel one and channel two of the Holter recording. The underlying rhythm has no obvious P waves with an undulating baseline, irregularly irregular R—R intervals (approximate HR of 120 bpm) and a narrow QRS complex consistent with AF. A prolonged series (232 QRS complexes) of alternating narrow and wide QRS complexes with a relatively consistent coupling interval (approximately 0.31 s) was noted consistent with ventricular bigeminy [6].
In general, abnormally wide QRS complexes during AF can be caused by ventricular premature complexes (VPCs), ventricular enlargement, or by aberrantly conducted supraventricular beats [7]. A relatively constant coupling interval to the preceding beat, consistent left bundle branch morphology on channel two and an opposite initial vector direction compared with the narrow QRS complex in channel two suggests that these wide QRS complexes are likely to be ventricular in origin [7]. Some of these wide QRS complexes do not have a complete compensatory pause as would be expected from a VPC [8]. However, absence of a compensatory pause after a VPC is possible due to retrograde activation of the sinoatrial node or if the PVC is interpolated between two supraventricular beats with out affecting the underlying rhythm [8]. On channel one, these wide QRS complexes occasionally appears to change mor¬phology and vector direction. This may be due to multifocal origin of VPCs or due to VPCs being conducted in a different pathway causing changes in the QRS morphology [9] The run of ventricular bigeminy was terminated by a wide QRS complex (black arrow in Fig. 2B) with an instantaneous HR of 68 bpm. This QRS complex may represent a supraventricular beat with a conduction block such as a bundle branch block, a fusion beat, or a late diastolic VPC occurring just before the next expected supraventricular beat [9].
It is difficult to characterize the exact origin of this QRS complex since channels in a Holter recording do not represent any specific lead morphology. A premature beat with a short coupling interval (0.22 s) is noted to be superimposed on the descending limb of the T wave of this QRS complex. A VPC occurring during the repolarization period (T wave) of the previous beat is known as 'R on T phenomenon’ [10]. The R on T VPC appears to initiate a polymorphic ventricular tachycardia (PVT) or ventricular flutter (with an approximate ventricular rate of 400—600 bpm) that rapidly degenerates into coarse ventricular fibrillation.
Table 1 Comparison of heart rate, QTAF interval, and ventricular arrhythmia status between in hospital electrocardiogram and Holter recordings obtained after antiarrhythmic treatment in a dog diagnosed with severe subaortic stenosis and atrial fibrillation.
Figure 2 (A) Representative portion of the Holter recording (tracing from two channels) obtained at the time of sudden death from a dog previously diagnosed with severe subaortic stenosis
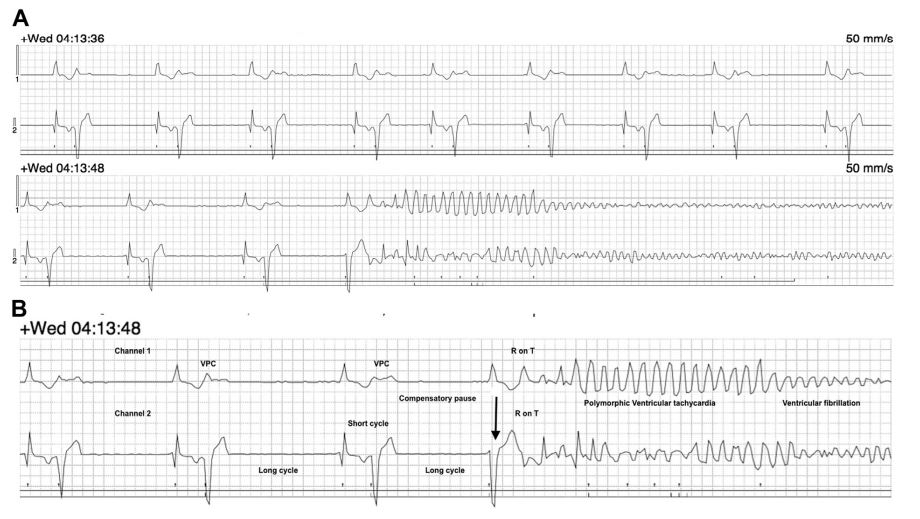
The underlying rhythm is consistent with atrial fibrillation with a mean heart rate of 120 bpm. Notice the prolonged series of ventricular premature complexes in bigeminal pattern alternating with the supraventricular complexes. Paper speed 50 mm/s; 1cm = 1 mV. (B) Magnified image focused on the arrhythmia immediately preceding sudden cardiac death from a dog previously diagnosed with severe subaortic stenosis . Notice that the ventricular bigeminy rhythm is terminated by a wide complex QRS complex beat (black arrow). An R on T ventricular premature complex superimposed on the T wave of the preceding QRS complex initiates polymorphic ventricular tachycardia or ventricular flutter that immediately degenerates into ventricular fibrillation. Paper speed = 50 mm/s; 1 cm = 1 mV.
Discussion
Sudden cardiac death is a known risk in dogs with arrhythmias secondary to various structural heart diseases [11]. It is also a frequent outcome in dogs with certain inherited arrhythmias such as arrhythmogenic right ventricular cardiac myopathy and German Shepherd dog inherited ventricular arrhythmia [11,12]. However arrhythmic events preceding SCD and the risk factors for the development of SCD are not well defined in dogs. In a report of people who experienced SCD while wearing a Holter monitor, the majority of SCDs (73%) resulted from ventricular tachyarrhythmias with ventricular tachycardia (VT) degenerating into ventricular fibrillation (VF) being the most common arrest rhythm [13]. Bradyarrhythmia-associated SCD were noted in 26% of the patients [13].
Ventricular premature complexes occurring within the preceding T wave were noted as an electrical event preceding SCD in about 70% of the patients that experienced sudden onset primary VF in the above study [13]. Interestingly, R on T phenomenon precipitating PVT that degenerated into VF was noted immediately preceding SCD in this dog. Ventricular premature complex with R on T phenomenon describes the superimposition of the ventricular depolarization on the previous repolarization wave (T wave) [10]. In humans, superimposition of the R wave can happen on the ascending limb of the T wave, on top of the T wave, or more commonly on the descending limb (61%) of the T wave [14]. The descending limb of the T wave represents transmural dispersion of repolarization and electrical stimulation during this period and is thought to be more likely to induce re-entrant arrhythmias [15]. Due to this potential to induce fatal arrhythmias, R on T VPCs were graded as a high-grade arrhythmia (Lown’s classification [9] grade 5 out of 5) necessitating antiarrhythmic therapy [16]. However, subsequent studies challenged the ability of R on T VPCs to consistently induce VF or sustained VT [17,18]. Though the potential of R on T to consistently induce VF is debatable, their frequent occurrence in acute MI following myocardial ischemia is beyond dispute [19]. In dogs, ante-mortem diagnosis of major coronary artery MI is rare [20]. However, it has been documented during postmortem examination in dogs with severe SAS [20]. The mechanism of SCD in patients with SAS remains unclear, but myocardial ischemia resulting from altered perfusion is thought to be a potential cause of fatal arrhythmias in dogs with severe SAS [21].
It is possible that myocardial ischemia resulting from severe SAS could have provided electrical substrate for initiation and maintenance of ventricular arrhythmias in this dog.
In the absence of MI, R on T VPCs are also frequently noted to initiate torsade de pointes (TdP) in people with long QT syndrome (LQTS) [22]. Prolongation of the QT interval is a known risk factor for SCD in people and this dog’s QT interval on the day of SCD showed mild QT prolongation compared with the average QT interval calculated from the previous Holter recording (Table 1). The QT interval correction in this report was calculated using a recently proposed method for QT correction in AF (QTAF = 0.126*(1-RRmod) + QT, where RRmod = (5*RR1 + 2*RR2 + RR3 + RR4 + RR5)/10) [1].
This method accounts for the effect of remote and varying RR cycle lengths on the QT interval (QT lag) and provides rate independent correction for QT interval [1]. In addition, the QRS complex (black arrow in Fig. 2A) where R on T occurred had more significant prolongation in QT interval (0.32 s; QTc = 0.38 s calculated using Fridericia’s formula [23] [QT/RR1/3] since the QTAF method cannot be applied during VA). The abnormally prolonged repolarization period due to prolonged QT interval may give rise to early depolarizations leading to triggered activity and initiate a specific form of PVT called TdP [24]. In human patients, prolonged QT interval can occur due to inherited ion channelopathies (congenital LQTS), electrolyte disturbances, or from drug therapy (acquired LQTS) [22].
In dogs, both acquired and congenital LQTS has been previously reported [24,25]. Tor¬sade de pointes resulting from acquired LQTS has many characteristic features on a surface ECG. Usually it is preceded by a short-long-short pattern of R—R interval cycles consisting of a VPC followed by a compensatory pause which is then terminated by a sinus beat, followed by a VPC occurring near the peak of the T wave (R on T phenomenon) of the previous sinus beat [26]. Though this dog shared similar ECG features (Fig. 1) of acquired TdP, (i.e. short—long cycle lengths and Ron T) the PVT noted in this dog was not long enough to evaluate for the characteristic appearance of the TdP. Also the Ron T in the acquired form of TdP is not typically tightly coupled compared with the early coupling of the R on T noted in this dog [26]. The rate control drugs that this dog (digoxin and diltiazem) was receiving at the time of SCD are not known to cause significant QT prolongation and therefore not likely to cause acquired LQTS [22]. Electrolyte disturbances such as hypokalemia, hypocalcemia, or hypomagnesemia resulting from concurrent furosemide use may result in acquired LQTS [22] however these values were not assessed in this dog, so its role if any is uncertain. Overall, it is unclear if the mild QT prolongation noted in this dog played any role in SCD in this dog. Also it should be clearly noted that QT measurements obtained from a Holter recordings are less accu¬rate compared with a standard ECG and is prone to observer related errors [27].
In this dog, despite improvement in clinical symptoms and initial improvement in VA status during therapy, a marked increase in the number of VPCs was observed in the Holter recording obtained at the time of SCD (Table 1). The observed increase in VA frequency was more than the previously reported 80% spontaneous variability for VA frequency in Boxer dogs with arrhythmogenic right ventricular cardiac myopathy [28]. New or worsening of ventricular ectopic activity in AF dogs with previously well-controlled HR should increase the suspicion of drug-induced dysrhythmia [29]. Of the two rate control drugs, this dog was receiving digoxin before SCD, which has the potential to induce arrhythmias even under normal therapeutic concentrations [30]. In this dog, a dramatic increase in the frequency of ventricular bigeminy was noted (Table 1). In human patients with con¬firmed digoxin toxicity, ventricular bigeminy is frequently noted and has been reported in about 24% of human patients with confirmed digoxin toxicity [31].
While serum digoxin levels were not assessed in this dog, despite receiving a low dose of digoxin, it was not exhibiting any systemic signs of toxicity consistent with acute toxicity. As such, it is unclear at this time if this dog was experiencing digoxin toxicity. In human patients, cardiac sensitivity to digoxin is increased when they are exposed to precipitating factors such as hypokalemia, hypomagnesemia, or hypothyroidism, even under normal serum digoxin levels [32].
A case-controlled large population—based study in people provided empirical evidence that digoxin—diuretic interactions increased the risk of hospitalization for digoxin toxicity in congestive heart failure patients [33]. The risk was particularly high for concomitant use of digoxin with a combination of loop diuretics, followed by thiazide and potassium-sparing diuretics. It is known in people that long-term digoxin users often have hypokalemia or hypomagnesemia, presumably due to diuretic usage in patients with congestive heart failure [33]. Unfortunately, serum electrolyte levels were not available during diu¬retic therapy for this dog but the role of electrolyte disturbances resulting from concomitant diuretic use potentiating digoxin-induced arrhythmias should be considered.
To the authors’ knowledge, this is the first case report in a dog where SCD is documented during Holter monitoring. For the dog in this report, lack of availability of blood work monitoring and thoracic radiographs was a major limitation in over all case management. It is likely that a combina¬tion of various factors including severe underlying heart disease, electrolyte abnormalities, and drug therapy played a role in the worsening ventricular arrhythmia and SCD in this dog. Clinicians must be aware of electrolyte abnormalities; drug interactions and pro-arrhythmic potential of commonly used drugs and discuss the risks with the clients before initiating therapy.
Conflicts of Interest Statement
The authors do not have any conflicts of interest to disclose.
References
- Saluja D, Guyotte J, Reiffel JA. An improved QT correction method for use in atrial fibrillation and a comparison with the assessment of QT in sinus rhythm. J Atr Fibrillation 2008;16:1—9.
- Rishniw M, Erb HN. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429—35.
- Boon J, Wingfield W, Miller C. Echocardiographic indices in the normal dog. Vet Radiol 1983;24:214—21.
- Francesco Porciello. Monodimensional echocardiography. In: Echocardiography, Cat, Dog and Horse; 2009. Accessed Online via the Veterinary Information Network URL: vin.com/members/cms/project/ defaultadv1.aspx?id=3863140&pid=11229.
- Brown DJ, Knight DH, King RR. Use of pulsed-wave Doppler echocardiography to determine aortic and pulmonary velocity and flow variables in clinically normal dogs. Am J Vet Res 1991;52:543—50.
- Wang Kyuhyun, Asinger Richard W, Marriott Henry JL. Bigeminal rhythms, common and uncommon mechanisms. J Electrocardiol 2007;40:135—8.
- Gulamhusein S, Yee R, Ko PT, Klein GJ. Electrocardio¬graphic criteria for differentiating aberrancy and ven¬tricular extrasystole in chronic atrial fibrillation: validation by intracardiac recordings. J Elecrocardiol 1985;18:41—50.
- Surawicz B, Knilans KT. Torsade de Pointes, ventricular fibrillation, and differential diagnosis of wide QRS tachycardias. In: Chou’s Electrocardiography in Clinical Practice (Sixth Edition). Adult and Pediatric. Philadelphia: WB Saunders; 2008. p. 440—54.
- Surawicz B, Knilans KT. Ventricular arrhythmia. In: Chou’s Electrocardiography in Clinical Practice (Sixth Edition). Adult and Pediatric. Philadelphia: WB Saunders; 2008. p. 405—39.
- Smirk FH. R waves interrupting t waves. Br Heart J 1949; 11:23—36.
- Basso C, Fox PR, Meurs KM, Towbin JA, Spier AW, Calabrese F, Maron BJ, Thiene G. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation 2004;109:1180—5.
- Moise NS, Meyers-Wallen V, Flahive WJ, Valentine BA, Scarlett JM, Brown CA, Chavkin MJ, Dugger DA, Renaud- Farrell S, Korneich B. Inherited ventricular arrhythmias and sudden death in German Shepherd Dogs. J Am Col Cardiol 1994;24:233—43.
- Watanabe E, Tanabe T, Osaka M, Chishaki A, Takase B, Niwano S, Watanabe I, Sugi K, Katoh T, Takayanagi K, Mawatari K, Horie M, Okumura K, Inoue H, Atarashi H, Yamaguchi I, Nagasawa S, Moroe K, Kodama I, Sugimoto T, Aizawa Y. Sudden cardiac arrest recorded during Holter monitoring: prevalence, antecedent electrical events, and outcomes. Heart Rhythm 2014;11:1418—25.
- Fries R, Steuer M, Schafers HJ, Bohm M. The R-On-T phe¬nomenon in patients with implantable cardioverter-defibrillators. Am J Cardiol 2003;91:752—5.
- Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 1998;98:1928—36.
- Gulierrez MR, Changfoot GH, Pereir DI. Significance of T wave interruption by premature beats as a cause of sudden death. Can Med Assoc J 1968;98:144—9.
- Bigger JTJ, Weld FM. Analysis of prognostic significance of ventricular arrhythmias after myocardial infarction. Shortcomings of Lown grading system. Br Heart J 1981;45: 717-24.
- Chou T, Wenzke F. The importance of R-on-T phenomenon. Am Heart 1978;96:191-4.
- Huikuri HV, Raatikainen MJ, Moerch-Joergensen R, Hartikainen J, Virtanen V, Boland J, Anttonen O, Hoest N, Boersma LV, Platou ES, Messier MD, Bloch- Thomsen PE, Cardiac Arrhythmias and Risk Stratification after Acute Myocardial Infarction study group. Prediction of fatal or near-fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction. Eur Heart J 2009;30: 689-98.
- Liu SK, Fox PR, Kirk RW. Myocardial ischemia and infarction. In: Current Veterinary Therapy XI. Philadelphia: W. B. Saunders; 2003. p. 791-5.
- Kienle RD, Thomas WP, Pion PD. The natural clinical history of canine congenital subaortic stenosis. J Vet Intern Med 1994;8:423-31.
- Abrams DJ, MacRae CA. Long QT syndrome. Circulation 2014;129:1524-9.
- Edwards NJ. Basic steps. In: Bolton - Handbook of Canine and Feline Electrocardiography. 2nd ed. Philadelphia: W.B. Saunders; 1987. p. 51-2.
- Melissa FR, James LD, Robert Jr GF, Freeman LC. Structural and functional basis for the long QT syndrome: relevance to veterinary patients. J Vet Intern Med 2003;17:473-88.
- Ware WA, Reina-Doreste Y, Stern JA, Meurs KM. Sudden death associated with QT interval prolongation and KCNQ1 gene mutation in a family of English Springer Spaniels. J Vet Intern Med 2015;2:561-8.
- Kay GN, Plumb VJ, Arciniegas JG, Henthorn RW, Waldo AL. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol 1983;2:806-17.
- Christiansen JL, Guccione P, Garson Jr A. Difference in QT interval measurement on ambulatory ECG compared with standard ECG. Pacing Clin Electrophysiol 1996;19:1296-303.
- Spier AW, Meurs KM. Evaluation of spontaneous variability in the frequency of ventricular arrhythmias in boxers with arrhythmogenic right ventricular cardiomyopathy. J Am Vet Med Assoc 2004;224:538-41.
- Chou TC. Effect of drugs on the electrocardiogram. In: Chou TC, editor. Electrocardiography in Clinical Practice. 3rd ed. Philadelphia: WB Saunders; 1991. p. 459-85.
- Kittleson M. Management of heart failure - pharmacoki¬netics. In: Kittleson M, Kienle R, editors. Small Animal Cardiovascular Medicine. 2nd ed. 2010 Accessed Online via the Veterinary Information Network. URL: vin.com/doc/?id=5496605&pid=5928.
- Irons Jr GV, Orgain ES. Digitalis induced arrhythmias and their management. Prog Cardiovasc Dis 1966;8:539-69.
- Dec GW. Digoxin remains useful in the management of chronic heart failure. Med Clin North Am 2003;87:317-37.
- Wang MT, Su CY, Chan AL, Lian PW, Leu HB, Hsu YJ. Risk of digoxin intoxication in heart failure patients exposed to digoxin-diuretic interactions: a population-based study. Br J Clin Pharmacol 2010;70:258-67.
^Наверх









