Biomarkers in cardiovascular disease: beyond natriuretic peptides

Author information
Boswood A. Biomarkers in cardiovascular disease: beyond natriuretic peptides // J Vet Cardiol. 2009 May;11 Suppl 1:S23-32.
Abstract
Currently natriuretic peptides are justifiably regarded as the most promising circulating markers of cardiovascular disease in dogs and cats, but there are many other markers that can be used in the evaluation of such patients. There are markers of myocyte injury typified by troponins I and T; markers of myocyte stress including adrenomedullin and ST2; markers of remodeling including matrix metalloproteinases, tissue inhibitors of metalloproteinases and collagen molecules (PIIINP); markers of endothelial function including dimethylarginines and nitric oxide metabolites; markers of inflammation including C-reactive protein, several interleukins and tumor necrosis factor alpha; and finally neurohormonal markers. The potential of many of these markers has at best been only partially explored in veterinary patients. Evidence emerging from studies of human and in some cases veterinary patients suggests that using multiple markers may be superior to using single markers alone. As well as evaluating markers for their diagnostic value they should be considered as methods of identification of patients at increased risk of experiencing complications or death. Future areas of research in this field could include improved characterization of the clinical utility of multi-marker evaluation in veterinary patients and using markers to identify patients that may benefit from particular interventions.
Introduction
Over the last two decades there has been a proliferation of literature regarding detectable circulating markers of cardiovascular disease and heart failure in human and veterinary patients. A recent article describing guidelines for the clinical utilization of such markers made the following bold statement ‘‘Biochemical marker testing has revolutionized the approach to diagnosis and management of heart failure over the past decade. There is an unsurpassed excitement in the heart failure community that significant advances in our understanding of currently available and future cardiac biomarkers will facilitate improved characterization of heart failure disease states and promote individualized therapy in heart failure and beyond.’’1
One working definition of biomarkers was suggested as follows: a biomarker is "a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.2’’ This could cover many diverse measurable characteristics in patients with heart disease. In this article I shall predominantly restrict my discussion to blood borne factors. Parameters routinely measured as part of standard laboratory investigations have been shown to have predict outcome in populations of human patients including concentrations of hemoglobin,3 creatinine,4 albumin5 and sodium.6 Concentrations of some of these substances are known to change in response to heart failure and its treatment in veterinary patients,7,8 and further study of these factors is warranted. I will however limit my discussion to factors not routinely measured on laboratory profiles, but measured specifically for the purpose of gaining insight into cardiovascular disease (Table 1). Much of the recent literature in both human and veterinary patients has justifiably concentrated on the evaluation of natriuretic peptides from the point of view of their value in diagnosis, prognosis, and guiding therapy. There are however many other established and emerging circulating markers that can be used to investigate and further characterize patients with cardiovascular disease. There are data regarding some of these markers in veterinary patients. In this article I aim to review some of the available markers, particularly, but not exclusively, those that have been evaluated in veterinary patients. A review of this area cannot possibly be comprehensive. There are simply too many available markers to allow for every one to be included. I have tried therefore to concentrate on those that have demonstrated the greatest potential in human patients and those which are considered to be the archetypes for each of the categories in which they will be considered.
We need a framework within which to assess the potential utility of biomarkers. It has been suggested that potential clinical applications of markers include the following 9:
- Detection of sub-clinical diseases
- Diagnosis of acute or chronic syndromes
- Risk stratification of patients
- Monitoring disease progression or response to therapy
- Selection of therapy.
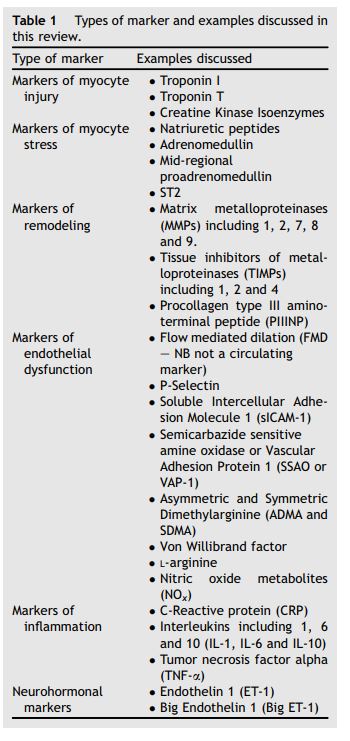
Table 1 Types of marker and examples discussed in this review.
I will try to restrict my discussion of biomarkers to those for which evidence of potential clinical utility in at least one of the above categories has been demonstrated. As new markers emerge, they tend first to be evaluated for their potential in cross-sectional studies which will enable a marker to qualify as useful in the first and second categories above. Evidence of utility in the third to the fifth category above require longitudinal studies and is therefore more difficult to demonstrate. Thus the longer a marker has been available the more likely it is that its value will have been completely characterized.
In a recent review article Braunwald proposed a system for categorizing biomarkers used in heart failure.10 I shall modify this system slightly and consider markers in the following categories:
- Markers of myocyte injury
- Markers of myocyte stress
- Markers of remodeling
- Markers of endothelial dysfunction
- Markers of inflammation, and
- Neurohormonal markers.
Markers of myocyte injury
- Although a variety of markers of myocyte injury have been used for the investigation of human patients with cardiac disease, only measurement of circulating serum, plasma or whole blood concentrations of cardiac troponins I and T has been widely used in veterinary patients. Prior to the availability of troponins, markers of myocyte injury were not widely used although the use of creatinine kinase for the identification of muscular and myocardial injury was described.11
- Troponins I and T are intracellular proteins that, with the assays currently available, are not normally present in detectable concentrations in the circulation. It is estimated that fewer than 1% of the normal human population will have detectable concentrations of circulating troponin.12 The frequency with which detectable concentrations can be found circulating in normal dogs and cats appears to be higher than that in normal human patients,13 but the normal concentrations that are found remain very low. Currently the principal clinical use of concentrations of troponins in human patients is for the detection of myocardial ischemia secondary to coronary vascular disease, primarily atherosclerosis.14
- This primary indication for the use of troponin is not a condition that occurs commonly in veterinary patients. However troponins have been measured in a multitude of different situations in dogs and cats to evaluate the effects of both cardiac and extracardiac diseases on myocardial integrity.15-24 In many of the situations in which it has been evaluated, troponin has been found to be elevated and therefore it has been interpreted to be a sensitive and specific marker of myocardial injury. In some of these studies histological confirmation of myocardial injury has been undertaken,16 and in isolated cases elevated troponin concentrations have been shown to occur accompanying histological evidence of myocarditis.25
- Considering the criteria by which a biomarker should be judged outlined above; as circulating markers, troponins I and T are able to detect ongoing myocardial injury in dogs associated with multiple diseases. However they non-specifically identify evidence of myocardial damage rather than identifying a particular cause of the damage, limiting their utility as tests for discrimination between causes of myocardial injury. In dogs with heart failure some studies have suggested a moderate ability to indicate the severity of disease,26 but these findings have been questioned by results obtained from larger studies.17 A study of over four thousand human heart failure patients demonstrated that a significantly greater proportion of those with detectable concentrations of cardiac troponin have more advanced failure (NYHA class III and IV) using both old and new, high-sensitivity assays.27
- These results still suggest that the ability of either assay to discriminate between classes of heart failure was poor. In cats with primary myocardial disease several studies have suggested troponin is a good indicator of the presence of disease,15,19 although it is likely that it will be superseded in this respect by natriuretic peptides which appear to have excellent discriminatory ability.28 Elevated troponin concentration has also been shown to discriminate cardiac from non-cardiac causes of dyspnea in cats reasonably effectively,29 but again it appears that N-terminal pro-brain natriuretic peptide (NTproBNP) may be more effective in this respect.28
- Troponin did not prove very discriminatory in screening dogs for evidence of occult myocardial disease,18 and one would think that the nonspecific nature of its elevation would result in a poor ability to screen for any particular cardiac disease in a diverse population. Oyama and Sisson17 demonstrated that the outcome for dogs with dilated cardiomyopathy which have elevated concentrations of troponin seems to be worse than for dogs with lower concentrations (Fig. 1)
- raising the possibility that there may be potential for prognostication or risk stratification with this test. It has also been proposed that in certain populations, for example dogs receiving pacemakers, an unusually elevated troponin concentration may indicate an animal at greater risk of having significant myocardial disease, and therefore one at greater risk of experiencing complications associated with the procedure, potentially justifying different treatment of such patients or more detailed diagnostic evaluation prior to implantation.25 Thus troponin concentrations may be of value for risk stratification in certain canine populations. There is little evidence in the veterinary literature of changes in troponin concentrations as indicators of response to therapy although one study showed a strong trend toward reduction in troponin concentrations in cats undergoing radioactive iodine treatment for hyperthyroidism.20 In this situation it might be that reduced troponin concentrations signify a resolution of detrimental effects of elevated circulating thyroid hormone concentrations on the myocardium.
- Recent developments in the assessment of circulating troponin concentrations in human patients suggest that further research in this area in veterinary patients is warranted. The generation of assays that have been used to measure troponin concentrations in the veterinary studies outlined above have all been plagued by a relatively high limit of detection. This means that many patients with mild to moderate disease had very low or undetectable levels of circulating troponin, complicating both interpretation of data and the statistical analysis of any results. High-sensitivity assays for use in people and animals27,30 have been developed with which the majority of individuals with even moderate disease have detectable concentrations. The elegant study by Latini and others27 showed that previously unmeasurable concentrations of troponin are highly predictive of outcome in a population of human patients with stable chronic heart failure of both ischemic and non-ischemic origin. They also showed that the predictive ability of this test is independent of, and complementary to, that achieved with concentrations of BNP. This raises the possibility of a multi-marker strategy, which in future might allow better identification of patients at risk of the worst outcomes.
Figure 1 Kaplan—Meier survival curves in dogs with cardiomyopathy
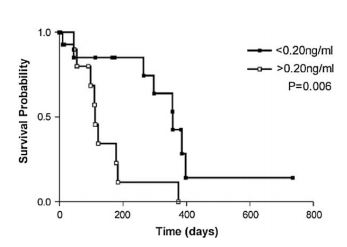
Dogs with cardiac troponin-I levels <0.20ng/mL (n = 15) had longer survival times than dogs with cardiac troponin-I levels >0.20 ng/mL (n = 11; P = .006; log-rank test). Reproduced from reference 17.
Markers of myocyte stress
The natriuretic peptides are considered to be markers of increased myocyte stress,10 but they are not the only ones. Increased myocardial stress is also associated with increased production of adrenomedullin and a soluble interleukin receptor referred to as ST2, reflecting the name of the gene which encodes it. As yet there are no peer- reviewed clinical studies evaluating concentrations of either of these markers in veterinary populations, however further study in this area is probably warranted.
ST2 is a circulating soluble form of a protein of the interleukin receptor family for which IL-33 may be the principal ligand.10 Concentrations increase incrementally with heart failure class,31 and increased concentrations are highly and independently predictive of one-year mortality.31 The predictive value complemented that of BNP and NTproBNP, again suggesting that a multi-marker strategy may be the best way forward for risk stratification in human, and by inference veterinary, populations.
Adrenomedullin is another peptide produced in increased quantities by cardiac myocytes under conditions of stress. Adrenomedullin concentrations have been shown to increase in dogs with experimentally induced heart failure.32 Concentrations of mid-regional proadrenomedullin, a stable peptide fragment derived from the precursor molecule of adrenomedullin, have been shown to independently predict outcome in human patients following myocardial infarction.33 Midregional proadrenomedullin concentrations provided additional information regarding prognosis in these patients adding to that derived from NTproBNP concentrations.
It is likely that further information about these markers in veterinary patients will emerge but currently they cannot be considered to have been shown to be clinically useful markers.
Markers of remodeling
Alterations of the constituents of the myocardium and myocardial mass accompany most significant cardiovascular diseases and are known broadly as remodeling. Matrix metalloproteinases (MMPs) represent a family of proteolytic enzymes involved in the regulation of myocardial extracellular matrix. A comprehensive review of their classification, manufacture, regulation and measurement has recently been published.34 Concentrations of MMPs can be measured in the plasma of patients with different cardiovascular diseases, as can concentrations of Tissue Inhibitors of Metalloproteinases (TIMPs). Several publications in human patients have shown that concentrations of these proteins and the ratios of concentrations of specific MMPs to specific TIMPs can provide additional information in populations with various diseases leading to ventricular remodeling. These include hypertension,35 acute myocardial infarction36 and dilated cardiomyopathy.37 The array of metalloproteinases measured in these studies and their inhibitors include MMPs 1,2, 7, 8 and 9 and TIMPs 1,2 and 4.
Although a fascinating role of MMPs in canine mitral valve disease has been suggested in acute remodeling experiments,38 they have yet to be systematically studied as circulating biomarkers of cardiovascular disease in veterinary patients.
One of the problems with these markers in the circulation is that they represent non-specific markers of connective tissue turnover that could potentially be occurring anywhere in the body. There are published studies evaluating alterations in MMP and TIMP concentration in the canine serum brought about by conditions as diverse as lymphoma, pregnancy, osteoarthritis and spinal injury.39-42 There is currently a lack of well conducted studies specifically evaluating the significance of these markers in naturally acquired cardiovascular disease and which, if any, of these markers will be of value in veterinary patients has yet to be established.
Another method by which turnover of connective tissue can be evaluated is to measure circulating concentrations of substances directly indicative of collagen turnover, including procollagen molecules. One interesting sub-analysis of the RALES study suggested that, in human patients, elevated serum concentrations of procollagen type III amino-terminal peptide (PIIINP) conferred a worse prognosis, but also indicated the population most likely to benefit from spironolactone therapy.43 More recent studies have also confirmed that in human patients increased concentrations are associated with a worse outcome.44 Serum PIIINP concentrations have been evaluated in a small group of dogs with cardiac disease.45 This study proved that PIIINP concentrations can be measured in dogs, but did not suggest any change in patients with cardiac disease. It is probably premature, on the basis of this single study, to conclude that measurement of these peptides will not be of value in dogs, but the initial results were not promising.
Markers of endothelial dysfunction
Endothelial dysfunction seems to occur in most cardiac and metabolic diseases of human patients and is in many circumstances linked to abnormalities of lipid metabolism and systemic inflammation (see next section). Several markers of endothelial function and dysfunction have been developed which can, in human populations, be predictive of risk of future adverse outcomes. Noninvasive evaluation of endothelial dysfunction in human patients with cardiovascular disease is performed both through direct measurement of vascular reactivity using flow mediated vasodilatation and indirectly through several circulating biomarkers of endothelial dysfunction.
Flow mediated vasodilatation (FMD) is currently the most widely used technique for demonstrating functional impairment of the vasculature in human patients.46 The predictive value of this marker in human patients has been established, for instance improvement of impaired FMD in response to treatment has been shown to be predictive of an improved outcome.47 The technique is technically demanding and although described in veterinary patients,48 its potential in evaluating patients with clinical disease has yet to be fully explored, although studies are ongoing.49
Many indirect markers of vascular endothelial dysfunction used for risk stratification have been developed and studied in human patients. These include P-selectin, C-reactive protein (CRP), soluble Intercellular Adhesion Molecule 1 (sICAM- 1), Semicarbazide Sensitive Amine Oxidase (SSAO, aka Vascular adhesion protein 1, VAP-1) and asymmetric dimethylarginine (ADMA) among others.50-53
In various studies these have been shown to be elevated in patients at risk of or currently suffering from cardiovascular disease. Although the conditions that typically lead to perturbations of these endothelial markers in human patients, e.g. atherosclerosis, type II diabetes and metabolic syndrome, are not recognized with the same frequency in veterinary patients, several studies have been performed to determine whether differences in circulating markers derived from the endothelium are altered in veterinary patients with cardiovascular disease. Their results have been mixed and sometimes conflicting with some suggesting changes in markers associated with cardiovascular diseases, and others failing to demonstrate differences. One published study suggested that markers of nitric oxide metabolism (NOx concentrations) were decreased in dogs with early mitral valve disease.54
A second study suggested that such markers might be increased in both dogs with mitral valve disease and dogs with dilated cardiomyopathy.55 A further study showed that the presence of cardiac disease did not seem to influence the concentration of dimethylargi- nines in dogs.56 Candidate markers of endothelial function and dysfunction in dogs can be considered to include NOx, von Willebrand’s factor, dimethy- larginines and L-Arginine,57 but no conclusive effect of cardiac disease has yet been demonstrated. A recent study demonstrated correlation between levels of circulating markers of endothelial function (dimethylarginines and NOx) and creatinine concentrations in cats with renal disease suggesting a possible association with endothelial dysfunction and renal failure, although there was no apparent association between the markers and elevated blood pressure.58 Although the above studies represent promising and valuable clinical research, incontrovertible evidence of generalized endothelial dysfunction in dogs or cats with heart failure has yet to be demonstrated. For this reason such markers cannot be considered to be of clinical value in veterinary patients at this time although they remain areas of active research.
Endothelin, a polypeptide hormone manufactured in the vascular endothelium will be considered below.
Markers of inflammation
Evidence of systemic inflammation co-existing with heart failure first emerged over half a century ago.59 Despite many years of active research the stimulus for this systemic inflammatory state in heart failure remains unclear and there are a number of competing hypotheses (Fig. 2); these include increased myocardial production of cytokines in response to chronic adrenergic stimulation, inflammation in response to tissue necrosis after myocardial infarction, tissue response to chronic underperfusion, and alterations in permeability and bacterial population in the underperfused gut.60-62 Irrespective of the elusive nature of the cause, what is not in doubt is that systemic inflammation mediates some of the detrimental long-term effects of chronic heart failure. Multiple circulating markers of inflammation have been described and variously used to characterize high risk populations. The first widely used marker was C-reactive protein (CRP) but other markers include interleukins 1, 6 and 10, and tumor necrosis factor alpha (TNF-a).51,60 In heart failure the detrimental effects of cytokines include further deterioration of myocardial function, remodeling, endothelial dysfunction and cachexia.60 Increased concentrations of cytokines have been shown to confer a worse outcome in human patients with heart failure.63
Figure 2 Competing theories. Several theories to explain the cause(s) of inflammatory immune activation in CHF have been suggested.
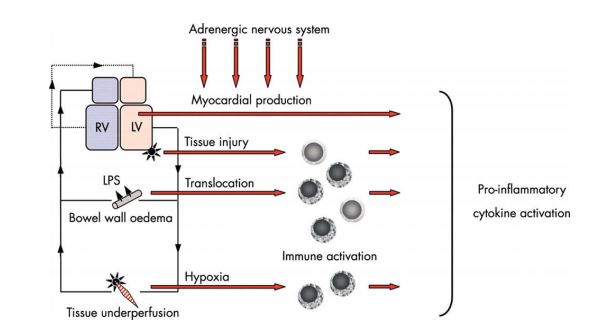
These theories may in fact complement each other. The myocardium itself is able to release proinflammatory cytokines, which is augmented by adrenergic stimulation. Myocardial tissue injury—for example, myocardial infarction, bacterial translocation, and peripheral tissue hypoxia—may lead to mononuclear cell activation, which eventually leads to pro-inflammatory cytokine activation. LPS, lipopolysaccharide; LV, left ventricle; RV, right ventricle. Reproduced from reference 60, Heart, Anker SD and von Haehling S., 90, 464-70, 2004 with permission from BMJ Publishing Group Ltd.
Therapy directed at intervening in the inflammatory process in heart failure has been attempted although results have been disappointing.64 Recent studies have however indicated that initiating statin therapy in high risk populations of human patients on the basis of elevated markers of vascular inflammation may be beneficial.65
Several clinical studies in veterinary patients with heart disease and heart failure have assessed, and in some circumstances shown significant alterations in, markers of inflammation in naturally occurring heart disease.8,55,66,67 One of these studies suggested that reduction of concentrations of interleukin-1 using dietary manipulation was associated with improvement in cachexia and prognosis.67 Thus there is some evidence of inflammatory stimulation in veterinary patients with heart failure and it may, as in humans, represent both a therapeutic target and an indicator of prognosis. Currently veterinary studies in this area are hampered by a lack of simple, validated species specific assays with low limits of detection. Similar to the problem with troponin described above, currently available assays do not measure appreciable circulating concentrations in many patients, and this is a limitation which needs to be overcome to facilitate further research in this promising area.
Neurohumoral markers
Heart failure has long been recognized as a state associated with altered circulating concentrations of neurohormones, these alterations being responsible for some of the detrimental outcomes in heart failure.68,69 Of all the circulating markers of neurohormonal activation the natriuretic peptides appear to be the most clinically valuable. Of the other markers endothelin-1 (ET-1), a hormone produced by the vascular endothelium, has also been assessed in dogs and cats.70,71 Some studies have suggested that although it is of moderate diagnostic value in veterinary patients it is not as discriminatory in this role as natriuretic peptides.24 It has also been suggested that in dogs an increasing concentration of a circulating marker of endothelin activity, Big ET-1, may be indicative of a worse outcome in dogs with dilated cardio- myopathy.72 In human patients elevated endothe- lin concentrations have been shown to be strong independent predictors of a poor outcome that provide information in addition to that given by natriuretic peptide concentrations.73
Challenges
What can be drawn from the bewildering and still incomplete list of biomarkers that has been discussed above? Our enthusiasm for current research in this field should not make us lose sight of the fact that only two markers of those that have been described above have genuinely been incorporated usefully into clinical practice and those are the natriuretic peptides and troponins.1
The clinical role of the other markers "remains to be determined and validated’’.1 There are currently multiple markers used in clinical research that have been shown to be capable of providing clinically useful information. There are therefore several challenges for the veterinary cardiology community.
The first challenge is to determine which marker or combination of markers is optimal for the circumstances in which it is to be applied. There is a lot that needs to be taken into consideration to answer this question and it requires prospective studies that assess the diagnostic and prognostic value of multiple markers simultaneously. Several studies of human patients have demonstrated that multi-marker strategies are worthwhile with combinations of markers providing information of greater value than any single marker alone.27,31 In addition to the scientific value of markers demonstrated in controlled studies, practical factors also need to be considered including the simplicity of sample handling and the accessibility of the assay.
The second challenge arising from studies of biomarkers is a generic challenge that emanates from any study where clinical information is derived from populations. Ultimately to be clinically valuable we must apply this information to individuals and therefore factors that seem clearly to confer a worse prognosis in a large population may still be of limited value in prognostication for a single patient.
The third challenge, particularly when dealing with information pertaining to prognosis is "what can we do about it?’’ Is there value simply in knowing that the outcome for particular patient is likely to be poor? Would it not be better to be able to change that outcome? The potential for us to use biomarkers to indicate which individuals within a population are likely to benefit from a particular intervention is an exciting development and an area that has yet to be explored in the veterinary field. The recently published JUPITER study65,74 enrolled otherwise healthy patients on the basis of elevated concentrations of CRP and demonstrated a benefit of therapy in this group. Perhaps the veterinary cardiology community should aspire to a study with similarly simple inclusion criteria, which would render the results easily and widely applicable to at risk patients.
The future
It is inevitable that with time our beliefs about the value of current biomarkers will be superseded as new biomarkers and new ways of characterizing disease become available to us. The search for new cardiovascular biomarkers for human patients is ongoing utilizing new biomedical research techniques including proteomics and metab- olomics.75 Hopefully some of the benefits anticipated in human medicine will spill over and create new opportunities in the veterinary field.
Conflict of Interest
Dr. Boswood has received support to perform biomarker assays from Guildhay, Guildford, UK and IDEXX Laboratories, Westbrook, ME, US. These supporters did not participate in any manner in the preparation of this manuscript.
References
- Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, Wu AH. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical utilization of cardiac biomarker testing in heart failure. Circulation 2007; 116:e99-e109.
- Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95.
- Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002;39:1780-1786.
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, Sutton GC. Survival of patients with a new diagnosis of heart failure: a population based study. Heart 2000;83:505-510.
- Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883-889.
- Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 1987;75:IV80-IV92.
- Boswood A, Murphy A. The effect of heart disease, heart failure and diuresis on selected laboratory and electrocardiographic parameters in dogs. J Vet Cardiol 2006;8:1-9.
- Farabaugh AE, Freeman LM, Rush JE, George KL. Lymphocyte subpopulations and hematologic variables in dogs with congestive heart failure. J Vet Intern Med 2004;18: 505-509.
- Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 2007;115: 949-952.
- Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148-2159.
- Aktas M, Auguste D, Concordet D, Vinclair P, Lefebvre H, Toutain PL, Braun JP. Creatine kinase in dog plasma: preanalytical factors of variation, reference values and diagnostic significance. Res Vet Sci 1994;56:30-36.
- Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA. Prevalence and determinants of troponin T elevation in the general population. Circulation 2006;113: 1958-1965.
- Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 2001;15:501-503.
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined - a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-969.
- Herndon WE, Kittleson MD, Sanderson K, Drobatz KJ, Clifford CA, Gelzer A, Summerfield NJ, Linde A, Sleeper MM. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002;16:558-564.
- Schober KE, Cornand C, Kirbach B, Aupperle H, Oechtering G. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation- volvulus. J Am Vet Med Assoc 2002;221:381-388.
- Oyama MA, Sisson DD. Cardiac troponin-I concentration in dogs with cardiac disease. J Vet Intern Med 2004;18:831 -839.
- Oyama MA, Sisson DD, Solter PF. Prospective screening for occult cardiomyopathy in dogs by measurement of plasma atrial natriuretic peptide, B-type natriuretic peptide, and cardiac troponin-I concentrations. Am J Vet Res 2007;68: 42-47.
- Connolly DJ, Cannata J, Boswood A, Archer J, Groves EA, Neiger R. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003;5:209-216.
- Connolly DJ, Guitian J, Boswood A, Neiger R. Serum troponin I levels in hyperthyroid cats before and after treatment with radioactive iodine. J Feline Med Surg 2005; 7:289-300.
- Hagman R, Lagerstedt AS, Fransson BA, Bergstrom A, Haggstrom J. Cardiac troponin I levels in canine pyometra. Acta Vet Scand 2007;49:6.
- Baumwart RD, Orvalho J, Meurs KM. Evaluation of serum cardiac troponin I concentration in boxers with arrhyth- mogenic right ventricular cardiomyopathy. Am J Vet Res 2007;68:524-528.
- Burgener IA, Kovacevic A, Mauldin GN, Lombard CW. Cardiac troponins as indicators of acute myocardial damage in dogs. J Vet Intern Med 2006;20:277-283.
- Prosek R, Sisson DD, Oyama MA, Solter PF. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B-type natriuretic factor, endo- thelin, and cardiac troponin-I. J Vet Intern Med 2007;21: 238-242.
- Church WM, Sisson DD, Oyama MA, Zachary JF. Third degree atrioventricular block and sudden death secondary to acute myocarditis in a dog. J Vet Cardiol 2007;9:53-57.
- Spratt DP, Mellanby RJ, Drury N, Archer J. Cardiac troponin I: evaluation I of a biomarker for the diagnosis of heart disease in the dog. J Small Anim Pract 2005;46:139-145.
- Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242-1249.
- Connolly DJ, Soares Magalhaes RJ, Syme HM, Boswood A, Luis Fuentes V, Chu L, Metcalf M. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med 2008; 22:96-105.
- Herndon WE, Rishniw M, Schrope D, Sammarco CD, Boddy KN, Sleeper MM. Assessment of plasma cardiac troponin I concentration as a means to differentiate cardiac and noncardiac causes of dyspnea in cats. J Am Vet Med Assoc 2008;233:1261-1264.
- Schultze AE, Konrad RJ, Credille KM, Lu QA, Todd J. Ultrasensitive cross-species measurement of cardiac troponin-I using the Erenna immunoassay system. Toxicol Pathol 2008; 36:777-782.
- Rehman SU, Mueller T, Januzzi Jr JL. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 2008;52:1458-1465.
- Jougasaki M, Grantham JA, Redfield MM, Burnett Jr JC. Regulation of cardiac adrenomedullin in heart failure. Peptides 2001;22:1841-1850.
- Khan SQ, O'Brien RJ, Struck J, Quinn P, Morgenthaler N, Squire I, Davies J, Bergmann A, Ng LL. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol 2007;49: 1525-1532.
- Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007;87:1285-1342.
- Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metal- loproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 2006;113:2089-2096.
- Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation 2006;114:1020-1027.
- Schwartzkopff B, Fassbach M, Pelzer B, Brehm M, Strauer BE. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur J Heart Fail 2002;4:439-444.
- Stewart Jr JA, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell'Italia LJ. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol 2003;35: 311-319.
- Gentilini F, Calzolari C, Turba ME, Agnoli C, Fava D, Forni M, Bergamini PF. Prognostic value of serum vascular endothelial growth factor (VEGF) and plasma activity of matrix metalloproteinase (MMP) 2 and 9 in lymphoma-affected dogs. Leuk Res 2005;29:1263-1269.
- Levine JM, Ruaux CG, Bergman RL, Coates JR, Steiner JM, Williams DA. Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am J Vet Res 2006; 67:283-287.
- Salinardi BJ, Roush JK, Schermerhorn T, Mitchell KE. Matrix metalloproteinase and tissue inhibitor of metalloproteinase in serum and synovial fluid of osteoarthritic dogs. Vet Comp Orthop Traumatol 2006;19:49-55.
- Schafer-Somi S, Ali Aksoy O, Patzl M, Findik M, Erunal- Maral N, Beceriklisoy HB, Polat B, Aslan S. The activity of matrix metalloproteinase-2 and -9 in serum of pregnant and non-pregnant bitches. Reprod Domest Anim 2005;40:46-50.
- Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales investigators. Circulation 2000;102:2700-2706.
- Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, Van Veldhuisen DJ, Tavazzi L, Mann DL, Cap- iaumont-Vin J, Li M, Hanriot D, Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail 2008;14:467-474.
- Schuller S, Valentin S, Remy B, Jespers P, Foulon S, Van Israel N, Clercx C, McEntee K. Analytical, physiologic, and clinical validation of a radioimmunoassay for measurement of procollagen type III amino terminal propeptide in serum and bronchoalveolar lavage fluid obtained from dogs. Am J Vet Res 2006;67:749-755.
- Al-Qaisi M, Kharbanda RK, Mittal TK, Donald AE. Measurement of endothelial function and its clinical utility for cardiovascular risk. Vasc Health Risk Manag 2008;4: 647-652.
- Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 2002;40: 505-510.
- Puglia GD, Freeman LM, Rush JE, King RG, Crawford SL. Use of a flow-mediated vasodilation technique to assess endothelial function in dogs. Am J Vet Res 2006;67:1533-1540.
- Jones ID, Luis Fuentes V, Fray T, Beyer S, Jones J, Vallance C, Elliott J. Flow mediated dilation in healthy dogs [abstract]. In: Proceedings of the eighteenth European society of veterinary internal medicine congress. Gent; 2008. p. 199.
- Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation 2005;112:1428-1434.
- Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res 2001;89:763-771.
- Boomsma F, de Kam PJ, Tjeerdsma G, van Den Meiracker AH, van Veldhuisen DJ. Plasma semicarbazide- sensitive amine oxidase (SSAO) is an independent prognostic marker for mortality in chronic heart failure. Eur Heart J 2000;21:1859-1863.
- Duckelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol 2007; 27:2037-2042.
- Pedersen HD, Schutt T, Sondergaard R, Qvortrup K, Olsen LH, Kristensen AT. Decreased plasma concentration of nitric oxide metabolites in dogs with untreated mitral regurgitation. J Vet Intern Med 2003;17:178-184.
- de Laforcade AM, Freeman LM, Rush JE. Serum nitrate and nitrite in dogs with spontaneous cardiac disease. J Vet Intern Med 2003;17:315-318.
- Pedersen LG, Tarnow I, Olsen LH, TeerlinkT, Pedersen HD. Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethy- larginines in dogs. Res Vet Sci 2006;80:336-342.
- Moesgaard SG, HolteAV, MogensenT, Molbak J, Kristensen AT, Jensen AL, TeerlinkT, Reynolds AJ, Olsen LH. Effects of breed, gender, exercise and white-coat effect on markers of endothelial function in dogs. Res Vet Sci 2007;82:409-415.
- Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, L- arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008;22:317-324.
- Elster SK, Braunwald E, Wood HF. A study of C-reactive protein in the serum of patients with congestive heart failure. Am Heart J 1956;51:533-541.
- Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart 2004;90:464-470.
- Niebauer J, Volk HD, KempM, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 1999;353: 1838-1842.
- SandekA, BauditzJ, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1561-1569.
- Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000;102: 3060-3067.
- Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594-1602.
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto Jr AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-2207.
- Rush JE, Lee ND, Freeman LM, Brewer B. C-reactive protein concentration in dogs with chronic valvular disease. J Vet Intern Med 2006;20:635-639.
- Freeman LM, Rush JE, Kehayias JJ, Ross Jr JN, Meydani SN, Brown DJ, Dolnikowski GG, Marmor BN, White ME, Dinarello CA, Roubenoff R. Nutritional alterations and the effect of fish oil supplementation in dogs with heart failure. J Vet Intern Med 1998;12:440-448.
- Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;341:577-585.
- Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract 2004;34:1105-1126.
- Prosek R, Sisson DD, Oyama MA, Biondo AW, Solter PE. Measurements of plasma endothelin immunoreactivity in healthy cats and cats with cardiomyopathy. J Vet Intern Med 2004;18:826-830.
- Prosek R, Sisson DD, Oyama MA, Biondo AW, Solter PF. Plasma endothelin-1 immunoreactivity in normal dogs and dogs with acquired heart disease. J Vet Intern Med 2004;18: 840-844.
- O'Sullivan ML, O'Grady MR, Minors SL. Plasma big endo- thelin-1, atrial natriuretic peptide, aldosterone, and norepinephrine concentrations in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2007;21:92-99.
- Selvais PL, Robert A, Ahn S, van Linden F, Ketelslegers JM, Pouleur H, Rousseau MF. Direct comparison between endothelin-1, N-terminal proatrial natriuretic factor, and brain natriuretic peptide as prognostic markers of survival in congestive heart failure. J Card Fail 2000;6:201-207.
- Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high- sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 2003;108:2292-2297.
- Gerszten RE, Wang TJ. The search for new cardiovascular biomarkers. Nature 2008;451:949-952.
^Наверх









