Intrapulmonary arteriovenous anastomoses in dogs with severe Angiostrongylus vasorum infection: clinical, radiographic, and echocardiographic evaluation

Author information
Novo Matos J., Malbon A., Dennler M., Glaus T. Intrapulmonary arteriovenous anastomoses in dogs with severe Angiostrongylus vasoruminfection: clinical, radiographic, and echocardiographic evaluation // J Vet Cardiol. 2016 Jun;18(2):110-24.
Abstract
BACKGROUND: In both humans and dogs the pulmonary vasculature is able to recruit large-diameter anatomical intrapulmonary arteriovenous anastomoses (IPAVAs) . In healthy people the opening of these anastomoses affects the degree of exercise-induced increase in pulmonary arterial pressure. The presence of these IPAVAs can be demonstrated using saline contrast echocardiography.
OBJECTIVES: The aims of the present study were to characterize severely affected, naturally infected dogs with Angiostrongylus vasorum, to evaluate if these dogs can open IPAVAs, and to assess if the recruitment of such anastomoses affects the severity of pulmonary hypertension (PH).
ANIMALS: Eight client-owned dogs with severe A. vasorum infection were recruited.
METHODS: Dogs with A. vasorum infection that presented with severe dyspnea and/or syncope were prospectively screened by echocardiography for the presence of PH and IPAVAs. Only severely affected dogs, based on a combination of clinical, radiographic and echocardiographic abnormalities, were enrolled.
RESULTS: Opening of IPAVAs could be demonstrated in three dogs with no to moderate PH, and could not be demonstrated in five dogs with severe PH. In two dogs thoracic radiographs showed only mild interstitial changes, while computer tomography and postmortem examination revealed severe pulmonary interstitial and vascular disease.
CONCLUSIONS: These results suggest that dogs may open IPAVAs and that opening of such anastomoses may play a regulatory role in the development of PH. There may be a marked discrepancy between radiographic changes and disease severity in A. vasorum.
KEYWORDS: Bubble study; Computed tomography; Contrast echocardiography; Intrapulmonary shunts; Pulmonary hypertension
Abbreviation Table
- AT acceleration time
- CT computed tomography
- ET ejection time
- IPAVAs intrapulmonary arteriovenous anastomoses
- LV left ventricle
- PAP pulmonary arterial pressure
- PG pressure gradient
- PH pulmonary hypertension
- PR pulmonic regurgitation
- PVR pulmonary vascular resistance
- RV right ventricular
- SCE saline contrast echocardiography
- sPAP systolic pulmonary arterial pressure
- TAPSE tricuspid annular plane systolic excursion
- TR tricuspid regurgitation
Introduction
The pulmonary vasculature is able to recruit large-diameter intrapulmonary arteriovenous anastomoses (IPAVAs) in healthy humans and dogs [1,2]. These are dynamic preformed silent vascular conduits that, when opened, create a bypass through the pulmonary microcirculation as these anastomoses allow arterial blood to bypass the capillary beds and join up with postcapillary venous blood [3]. The presence of such anastomoses can be demonstrated by contrast echocardiography [4].
There is anatomical [2,5,6] and biophysical [2,7,8] evidence of the presence of IPAVAs in humans and dogs. The ability to recruit intrapulmonary arteriovenous anastomoses varies between individuals, and not all humans or dogs, healthy or diseased, are able to open IPAVAs [1,2,7,9]. These physiological anastomoses are not to be confused with macroscopic arteriovenous-fistula malformations, even though the consequences may be similar [10,11].
Intrapulmonary arteriovenous anastomoses may be involved in both physiological and pathological conditions, although their precise role remains controversial [12,13]. One potential important role may be the regulation (decrease) of pulmonary arterial pressure (PAP) in health, e.g. during exercise, and disease. Specifically, intrapulmonary arteriovenous anasto¬moses may work as "pop-off valves” in the face of increased flow and pressure thus minimizing damaging effects on the pulmonary microcirculation and reducing right ventricular (RV) afterload [14].
Pulmonary hypertension (PH) is a hemodynamic abnormality of considerable and growing importance in dogs, Angiostrongylus vasorum being an increasingly recognized cause [12—17]. This parasite causes severe pulmonary vascular and parenchymal lesions initiated by an intense immune response against eggs and larval antigens in (peripheral) pulmonary arteries. In some dogs, widespread pulmonary thrombosis can be found predominantly in more peripheral, but sometimes even in the main pulmonary arteries. In other dogs, widespread essentially complete pulmonary parenchymal destruction with severe hemorrhage and loss of recognizable architecture is found. Finally, there is some pulmonary artery remodeling [18—21].
Thus, A. vasorum may induce increased pulmonary vascular resistance (PVR)and resulting PH by multiple mechanisms [22]. Considering the often severe and extensive nature of these pulmonary parenchymal and vascular lesions, PH would be expected to be more commonly found in this disease. Nevertheless, until very recently, reports of PH in dogs secondary to A. vasorum infection were scant, [12—15] and one recent case series described moderate-to-severe PH in only 14.6% of infected dogs in a referral population [17]. It is unclear why some individuals with severe pulmonary parenchymal and vascular lesions (including pulmonary arterial thrombosis) do not develop PH, while others with a similar severity of lesions will show clinically significant PH.
Using A. vasorum infection as a model of severe pulmonary vascular and parenchymal disease we had recently hypothesized that PAP may be influenced by the presence of intrapulmonary arteriovenous anasto¬moses; in fact, in an experimental study 2 dogs without identifiable IPAVAs showed a mild increase in PAP as opposed to 4 dogs with intrapulmonary arteriovenous anasto¬moses that did not show an increase [7]. Consequently, the individual ability to recruit IPAVAs may be one factor influencing the development of PH in A. vasorum infected dogs and, by affecting RV function, possibly prognosis.
The aims of the present study were (1) to characterize clinically, radiographically, echo- cardiographically and pathologically severely and naturally affected dogs with A. vasorum, (2) to evaluate by contrast echocardiography if these dogs can recruit IPAVA and (3) to assess a possible relationship between intrapulmonary arteriovenous anastomoses and the presence and severity of PH. The specific study hypothesis was that dogs without IPAVAs have more severe PH compared to dogs with these intrapulmonary anastomoses.
Animals, materials and methods
Client-owned dogs presented to the Small Animal Hospital of the Vetsuisse Faculty, University of Zurich with A. vasorum infection confirmed by a Baermann fecal exam were prospectively screened by echocardiography for the presence of PH and IPAVAs. Dogs were only included in the study if they had severe disease, which was defined as (1) presence of severe dyspnea and/or syncope plus (2) at least one of the following criteria: (a) combination of marked changes on thoracic radiographs, characterized by a moderate-to-severe generalized interstitial to alveolar pattern, or (b) severe PH on echocardiography (see definition in the following). Eight dogs met these criteria. All dogs were examined by thoracic radiographs and a complete echocardiographic examination includ¬ing saline contrast echocardiography (SCE). Additional individual tests included arterial blood gas analysis, and thoracic angiographic-computed tomography (CT).
Echocardiographic examinations were performed with continuous electrocardiographic monitoring on laterally recumbent unsedated dogs using a GE Vivid 7 ultrasound unit.d Two-dimensional, M-mode, color, pulsed wave, continuous wave, and tissue Doppler images were obtained from right and left parasternal standard views [23]. The primary focus was RV morphological and functional assessment and the presence and velocity of tricuspid valve regurgitation (TR) and/or pulmonic valve regurgitation (PR). The RV morphology was subjectively assessed by comparison with the left ventricular chamber size and wall thickness, as previously described [24].
The RV variables studied were peak velocity of pulmonary artery flow and systolic flow profile, and RV systolic time intervals (i.e. preejection period, acceleration time [AT], ejection time [ET], preejection period/ET ratio, and AT index [AT/ET ratio]) acquired from the right parasternal short- axis view at the level of the heart base with the pulsed wave Doppler sample volume in the midstream between the opened pulmonic valve leaflets where a clear laminar flow pattern was recorded. Systolic flow profiles were defined as type I, normal flow profile, symmetric envelope, equal acceleration and deceleration times; type II, peak velocity occurring early in systole with a longer deceleration time; type III, rapid acceleration with notching during deceleration [25].
Tricuspid annular plane systolic excursion (TAPSE) was measured from M-mode recordings of the lat¬eral tricuspid valve annulus from a left parasternal apical four-chamber view optimized for the RV, as previously described [26]. Whenever TR or PR was detected the Doppler beam was aligned as parallel as possible with the regurgitant flow in order to determine peak velocities and calculate peak pressure gradient (PG) using the modified Bernoulli equation (P = 4 x V^2) and estimate peak systolic and mean PAP, respectively. Estimated right atrial pressures were not added to the calculated TR gradient, but a fixed right atrial pressure was added for calculating PVR (see in the following) [27]. Pulmonary hypertension was defined as a peak TR >2.8 m/s (estimated systolic PAP >30 mmHg) and/or peak PR >2.2 m/s (estimated d Vivid 7, GE Medical Systems, Munich, Germany. mean PAP >20 mmHg) [24].
Dogs were further classified as having mild PH (peak systolic PG 30—50 mmHg), moderate PH (peak systolic PG 51—75 mmHg) and severe PH (peak systolic PG >75 mmHg) [24]. In cases in which no regurgitant jet was present hemodynamically significant PH was defined by the presence of RV hypertrophy/ dilatation, septal flattening, dilated main pulmonary artery in the absence of pulmonic stenosis and decreased left ventricle (LV) chamber size [28]. Pulmonary vascular resistance was calculated from estimated systolic pulmonary arterial pressure (sPAP) using a constant right atrial pressure of 8 mmHg (sPAP = 8 + 4 x TR2) and RV outflow tract time-velocity integral using the fol¬lowing formula: PVR = sPAP/RV outflow tract time- velocity integral + 3 if mid-systolic notch (type III) [27]. Pulsed wave tissue Doppler imaging was performed to evaluate RV longitudinal myocardial velocities at the tricuspid lateral annulus using a standard left apical four-chamber view. Peak annular systolic (S'), early (E') and late (A0) diastolic myocardial velocities were measured and the E'/A' ratio was calculated.
Table 1 Signalment, clinical and radiographic findings of eight dogs naturally infected with A. vasorum at baseline.
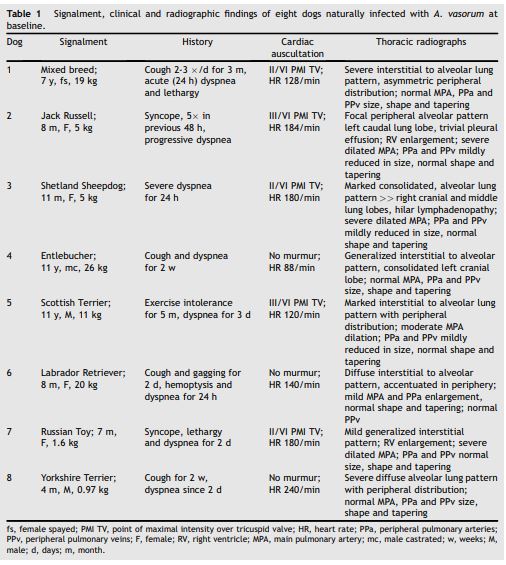
fs, female spayed; PMI TV, point of maximal intensity over tricuspid valve; HR, heart rate; PPa, peripheral pulmonary arteries; PPv, peripheral pulmonary veins; F, female; RV, right ventricle; MPA, main pulmonary artery; mc, male castrated; w, weeks; M, male; d, days; m, month.
All echocardiographic variables were measured at sweep speeds of 100 mm/s. Reported values represent the average of three measurements.
Contrast echocardiographic studies were performed from a standard right parasternal long-axis four-chamber view using hand-agitated saline injected into a cephalic vein as follows. An intra¬venous catheter was placed into the cephalic vein with a three-way stopcock attached. Two 5 mL Luer lock tip syringes were fixed to the three-way stopcock with 2—5 mL of sterile saline in one syringe and no air or fluid in the other. All air was also evacuated from the stopcock before producing the bubbles. Saline contrast bubbles were created by manually pushing the two syringe plungers back and forth until a homogeneous opaque solution was created. The saline-air microbubble suspension was then immediately injected by forced hand injection. If large bubbles were visible in a syringe during this procedure, these were removed prior to injection to minimize the risk of air embolism. Lower saline volumes (2 mL) were used in the dogs weighing less than 2 kg.
The presence of open intrapulmonary arteriovenous anasto¬moses was defined by the appearance of bubbles in the LV more than 3 cardiac cycles following contrast opacification of the right ventricle [8]. Intracardiac shunts (e.g. patent foramen ovale) are characterized by the appearance of saline contrast in the left heart almost coincident with right heart opacification, and appearance of bubbles in the LV <3 cardiac cycles after contrast opacification of the right ventricle [4]. Any oxygen therapy was discontinued 3 min before and while SCE was performed, and all dogs were in lateral recumbency during the contrast study [29]. Dogs were not receiving any therapy that could have an effect on pulmonary or systemic circulation (e.g. sildenafil or pimobendan) at the time of the echocardiographic exam. To assess the degree of intrapulmonary shunting a semiquantitative scoring system was used [30]. This grading system is composed of 5 scores where score 0 is the absence of bubbles in the LV in any frame, score 1 the presence of trace amounts of bubbles (<3 in any frame), score 2 a low amount of bubbles (4—12 bubbles in any frame), score 3 multiple isolated bubbles (>12 in any frame), score 4 numerous bubbles heterogeneously distributed through the LV, and score 5 when numerous bubbles are homogeneously distributed within the LV. A shunt score >2 is considered hemodynamically significant, i.e. able to affect PVR [31]. All SCEs were reviewed and scored by the same author (JNM).
For radiographic assessment two orthogonal projections of the thorax (right-to-left lateral and ventrodorsal) were obtained. In one dog (case 7) a thoracic angiographic-CT was performed to eluci¬date the marked discrepancy between severe clinical, blood gas and echocardiographic abnormalities and only mild radiographic pulmonary changes.
Partial (thoracic organs), and complete nec¬ropsies were carried out within 24 h of submission on cases 2 and 3 respectively with full owner consent. Tissue samples were fixed in 10% buffered formalin for 24—48 h and routinely embedded in paraffin wax before hematoxlin and eosin staining of 5 mm sections for histological examination.
Results
Clinical, laboratory and radiographic findings
Clinical and radiographic findings are summarized in Table 1. Overt right-sided congestive heart failure with ascites was present in only one dog (dog 5) and only developed after anti-parasitic treatment had been initiated. Arterial blood gas analyses were performed in three dogs (dogs 2, 3 and 7), and all three had severe hypoxemia (PaO2 43, 51 and 40 mmHg [reference range, 90—100 mmHg]; %SO2 83, 86 and 75% [reference range, >95%]), hypocarbia (PaCO2 25, 24 and 27 mmHg [reference range, 36—40 mmHg]) and respiratory alkalosis (pH 7.46, 7.45 and 7.46 [ref¬erence range, 7.36—7.44]).
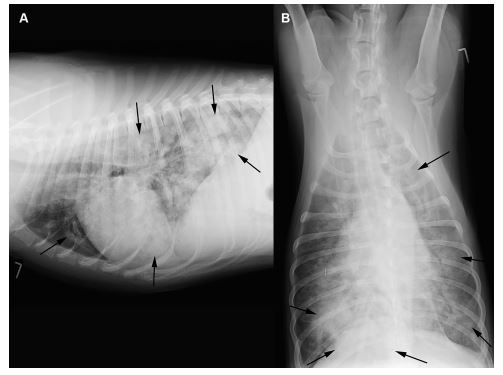
Thoracic radiographs showed mild (n = 2, Fig. 1), moderate (n = 2) or severe (n = 4, Fig. 2) interstitial to alveolar lung opacification, diffusely (n = 6) or multifocally (n = 2), mostly (n = 5) with peripheral predominance of the changes, typical for A. vasorum (Table 1). In none of the eight dogs were peripheral pulmonary arteries tortuous or ending abruptly. The main pulmonary artery was considered normal in three, mild-to-moderately enlarged in two, and severely enlarged in three dogs. In case 7, in contrast to the radiographs (Fig. 1), angiographic CT (Fig. 3) revealed severe pulmonary parenchymal disease. Specific CT abnormalities were multifocal, peripherally accentuated areas isoattenuating to soft tissues (10 ± 16 Hounsfield Units (HU)). Some of these wedge shaped areas contained air bronchograms while others had a more nodular character.
Figure 1 Right lateral (A) and ventrodorsal (B) thoracic radiographs of a dog infected with A. vasorum (dog 7).
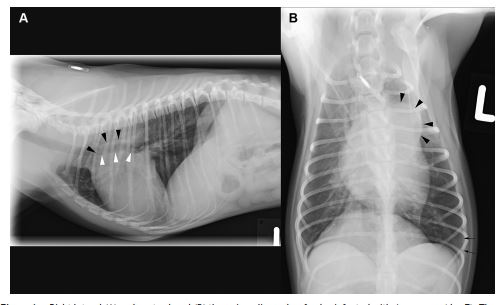
There is mild cardiomegaly and a severely dilated main and right pulmonary artery (arrow heads) with only a mild interstitial lung pattern, best visible in the periphery of the left caudal lung lobe (arrows).
Extensive areas of unstructured increase of pul¬monary attenuation with preserved bronchial and vascular markings (ground-glass opacification) sur¬rounded the peripheral lesions. Thin pleural fissure lines separated the mildly rounded lung lobes. The tracheobronchial lymph nodes were mildly enlarged. In the angiographic study irregularities of vascular diameter with sporadic filling defects occurred in the peripheral pulmonary arteries in close proximity to the pulmonary lesions.
Echocardiography
Echocardiographic data are reported in Table 2. Two-dimensional echocardiographic examination revealed structurally normal cardiac chambers in three dogs, two of which had mild-to-moderate (TR PG 44 and 52 mmHg) and one no demonstrable PH (no TR, PR PG 7 mmHg). In five dogs with severe PH there was subjective moderate-to-severe RV hypertrophy and underloaded LV. Right ventricular AT index and pulmonary artery systolic flow profile were obtained in seven dogs, and were normal in three dogs with mild-to-moderate or no PH (RV AT/ ET >0.31; all systolic flow profile type I) and abnormal in four dogs with severe PH (RV AT/ET <0.31, systolic flow profile types II and III in 2 dogs each).
Calculated PVR (n = 6) was elevated in all dogs, but markedly higher in dogs with severe PH. Systolic RV myocardial velocity (n = 5) was normal in all dogs, and TAPSE (n = 7) was normal in six dogs. Diastolic myocardial velocities were obtained in five dogs, including the three dogs with unremarkable two-dimensional images and none or mild-moderate PH, and in four of five dogs E'/A' was <1.
Contrast echocardiography
Intracardiac shunts were not observed in any of the 8 dogs on two-dimensional, color or SCE. Agitated saline contrast injection resulted in complete and homogenous RV opacification in each dog. No bubbles were detectable in the LV in any of the five dogs with severe PH (bubble score 0), but in all three dogs with no or only mild-moderate PH bubbles were seen in the LV four to five cardiac cycles after RV opacification (Table 2, Fig. 4, Video 1).
Outcome
Five dogs showed complete clinical recovery after treatment (dogs 1, 4, 6, 7 and 8) within the first 30 days of starting therapy. Dogs 2 and 3 died during the first 24 h of hospitalization and dog 5 was euthanized 4 months after presentation because of refractory right-sided congestive heart failure, despite successful elimination of A. vasorum.
Figure 2 Right lateral (A) and ventrodorsal (B) thoracic radiographs of a dog infected with A. vasorum (dog 6) showing a severe generalized interstitial to alveolar lung pattern (arrows) with normal cardiac dimensions.
Pathology
Necropsies were performed in dogs 2 and 3 in which gross and histological findings were very similar and therefore are described together. The lungs had a diffusely increased consistency and disseminated firm dark red nodules of 1—30 mm throughout the parenchyma, more prominent in the caudal lobes. There was moderate pulmonary edema and mild enlargement of bronchial lymph nodes. There was also subjective moderate RV wall hypertrophy with a dilated RV chamber and adult A. vasorum were present within the main pulmonary artery; multiple in case 2 and two in case 3.
Histologically, approximately 80% of the pul¬monary parenchyma was effaced by an inflammatory process associated primarily with A. vasorum L1 larvae, and lower numbers of eggs, within the alveoli and interstitium. These induced a granulomatous foreign body reaction and type 2 pneumocyte hyperplasia. In some places, but not as a dominant feature, the tunica media of both small and large arteries, and arterioles, was hypertrophied with activated nuclei; this was often seen in conjunction with degenerative changes including fibrinoid necrosis particularly of the smaller caliber vessels. Plexiform lesions were not observed. Multiple larger arteries contained adult nematodes while others were partially or entirely occluded by thrombi; in some cases these exhibited recanalization and contained nematode eggs and larvae (Fig. 5). Additionally there was acute subpleural hemorrhage and pleural fibrosis. Nematode larvae were present within the bronchial lymph nodes. No gross or histological abnormalities were detected in other organ systems (case 3).
Figure 3 Thoracic angiographic computed tomography images of the same dog as in Fig. 1 (dog 7).
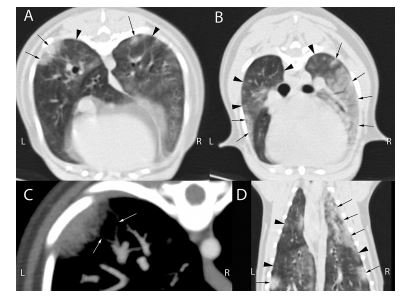
(A, B) Transverse images in pulmonary reconstruction and pulmonary window (window level [WL]/window width (WW): -500/1400) of the thorax; Panel A at the level of the accessory lung lobe, panel B cranial to the tracheal bifurcation. Peripherally distributed, wedge shaped or nodular lesions (black arrows) with adjacent areas of ground-glass opacification (black arrow heads) increase the attenuation of the lung. (C) Magnified maximum intensity projection of ten slices of the same region as A in the angiographic phase of contrast medium distribution (bolus tracking on the pulmonary artery) in soft tissue reconstruction and thromboembolism specific windowing (WL/WW: 100/700). Irregular narrowing of the lumen of the peripheral pulmonary arteries causes filling defects in the contrast distribution in close proximity to the pulmonary lesions (white arrows), suggesting pulmonary arterial thrombosis. (D) Dorsal reconstruction of the pul¬monary study with the same settings as A and B. The number and severity of pulmonary lesions with air bronchogram formation and adjacent ground-glass opacities were severely underestimated on the thoracic radiographs (Fig. 1).
Discussion
The results of the study corroborate our previous findings in dogs experimentally infected with A. vasorum that IPAVAs are not observed in all dogs [7]. More specifically, in dogs with severe PH IPA- VAs were not observed as opposed to dogs with only mild-moderate or no PH. This again correlates with our experimental study in which dogs with negative SCE results had higher PAP [7]. This seems to support our hypothesis that some dogs may not be able to recruit intrapulmonary arteriovenous anastomoses which may be associated with more severe PH; in other words recruitment of IPAVAs in dogs with pulmonary (vascular) disease may attenuate the development of PH.
In the present study only dogs with clinically significant A. vasorum infection defined by the presence of severe clinical signs and thoracic imaging results documenting severe disease were included. The rationale for this design was to only enroll dogs with a strong probability that pulmonary damage would be severe enough to cause severe hypoxia and/or PH, postulated stimuli to recruit IPAVAs [32,33]. In mild disease intrapulmonary arteriovenous anasto¬moses may not be recruited, even if the respective dog has the ability to recruit them, and in such a case a negative contrast echocardiography result would be judged as false negative.
In people, IPAVAs are believed to be remnants of fetal vessels and to physiologically play a role during fetal life by bypassing blood flow away from the non-functional pulmonary capillaries [9]. After birth, the three main potential consequences are regulation (decrease) of PAP, reduction of pulmo¬nary gas exchange efficiency, and loss of the pulmonary capillary filter. It has been shown that individuals able to open IPAVAs during exercise have lower PAP [34]. Isolated case reports have documented a hemodynamic and clinical benefit of macroscopic intrapulmonary arteriovenous malformations in diseased people with severe PH [35,36], similarly the ability to recruit IPAVAs may a Upper limit of normal for body weight written between parentheses. b Lower limit of normal for body weight written between parentheses.
Table 2 Selective echocardiographic findings in eight dogs naturally infected with A. vasorum
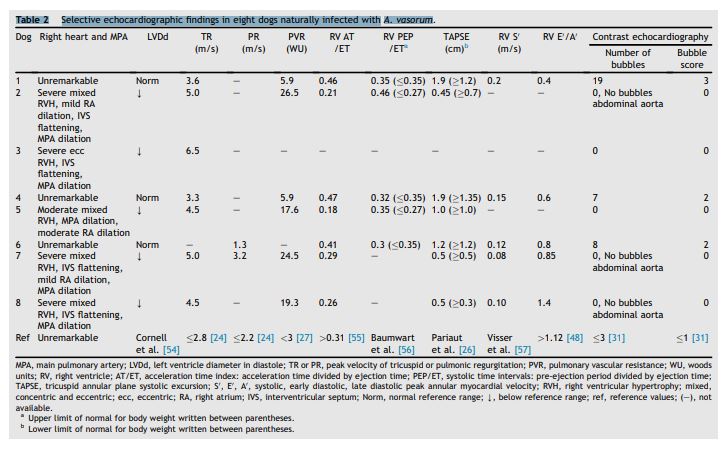
MPA, main pulmonary artery; LVDd, left ventricle diameter in diastole; TR or PR, peak velocity of tricuspid or pulmonic regurgitation; PVR, pulmonary vascular resistance; WU, woods units; RV, right ventricle; AT/ET, acceleration time index: acceleration time divided by ejection time; PEP/ET, systolic time intervals: pre-ejection period divided by ejection time; TAPSE, tricuspid annular plane systolic excursion; S', E', A', systolic, early diastolic, late diastolic peak annular myocardial velocity; RVH, right ventricular hypertrophy; mixed, concentric and eccentric; ecc, eccentric; RA, right atrium; IVS, interventricular septum; Norm, normal reference range; Y, below reference range; ref, reference values; (-), not available.4
Figure 4 Right parasternal long-axis four-chamber view saline contrast echocardiography obtained in a dog with A. vasorum infection
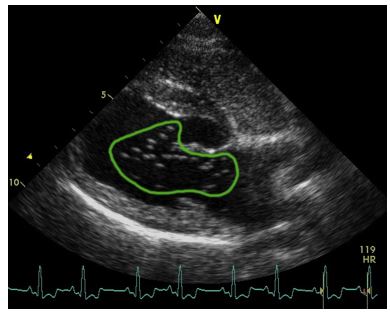
Figure 4 Right parasternal long-axis four-chamber view saline contrast echocardiography obtained in a dog with A. vasorum infection (dog 1) illustrating delayed right-to-left passage of saline contrast suggesting intrapulmonary arteriovenous anastomoses. Note the opacification of the right heart and the presence of bubbles in the left ventricle (bubble score 3; green line). The bubbles were identified in the left ventricle five cardiac cycles after they had reached the right ventricle. be an important defensive mechanism against PH in disease [37]. Whereas the PAP lowering effect may have advantages in health and disease, reduced gas exchange may worsen oxygenation which may contribute to exercise-induced arterial hypoxemia [38] and may be critical in severely hypoxic patients [39,40]. This effect is complex though, because a higher cardiac output by increasing mixed venous O2 content may compensate for the venous admixture caused by the IPAVA, and PaO2 may not change [33,34,41,42]. Finally, loss of capillary filter function may explain some forms of cryptogenic stroke by paradoxical embolism [9].
It is important to stress that intrapulmonary arteriovenous anasto¬moses are anatomic rather than functional shunts because only anatomic shunts have a sufficiently large diameter to allow bubbles to pass and reach the left heart, as contrast bubbles are significantly larger than pulmonary capillaries [1]. In cases of functional shunting (i.e. intrapulmonary shunting at the capillary level) as observed in ventilation-perfusion mismatch, bubbles are blocked at the capillary level (8—9 mm diameter) and thus do not bypass the lung circulation [34]. The exact size of IPAVAs in the canine lung is not known, but experimental work suggested these anastomoses to be between 25 and 420 mm in diameter [2,37,43].
The mechanisms regulating IPAVAs are not com¬pletely clear and there are conflicting results between studies [32,33,42]. In healthy humans it has been shown that the large majority of subjects open IPAVAs while breathing hypoxic gas mixtures with a fraction of inspired oxygen (FiO2) of 0.12 and 0.10 [8]. This differs from the usual behavior of the pulmonary circulation which vasoconstricts under low oxygen tension. It was even shown that hyperoxia leads to closure of IPAVAs or markedly attenuates the degree of shunting [30]. Based on these observations IPAVAs behave in the opposite manner to that of the rest of the pulmonary circu¬lation [44]. It is unknown how oxygen tension con¬trols intrapulmonary arteriovenous anasto¬moses, although the mechanism may be similar to that of the ductus arteriosus, which during fetal life in the hypoxic utero environment is patent and closes after birth with the increased oxygen tension [9].
Besides hypoxia, PAP may have a role on IPAVA recruitment as well. A positive correlation between pulmonary transit of agitated saline (bubble score) and systolic PAP has been shown, suggesting a possible effect of PAP on IPAVAs [9].
Figure 5 Histological sections of lung tissue from a dog with severe A. vasorum infection (dog 3).
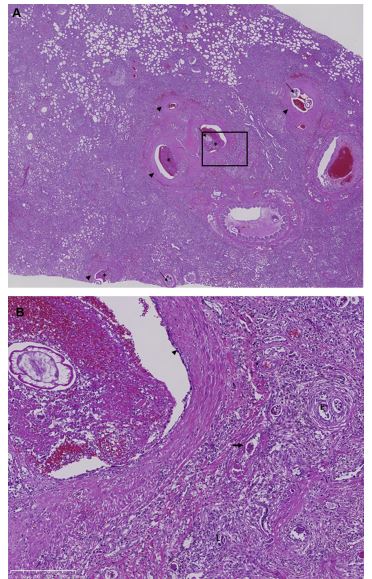
(A) Overview showing severe diffuse pulmonary consolidation and multifocal acute hemorrhages. Arterial hypertrophy (arrowheads), thrombi (*) and adult nematodes (arrows) are also visible. Hematoxylin and eosin, x1.5. (B) Higher magnification of marked area of image (A), adult nematode within a hyperplastic pulmonary artery exhibiting thrombus formation and endothelial cell activation (arrowhead). The surrounding parenchyma is consolidated by fibrosis and an inflammatory infiltrate of predominantly macrophages, lymphocytes and plasma cells with occasional giant cells, surrounding encysted eggs (E) and larvae (L). Additionally there is hyperplasia of small arterioles with multifocal fibrinoid necrosis of vessel walls (arrow). Hematoxylin and eosin stain, x10.
To document the presence of intrapulmonary arteriovenous anasto¬moses we used SCE, the usual method used in humans [1,4,9]. Saline contrast echocardiography is the most sen¬sitive technique in identifying right-to-left shunts, and is superior to high resolution CT in detecting microvascular pulmonary arteriovenous malformations [9]. The bubble scoring system is used as an attempt to quantitate the magnitude of blood flow through the intrapulmonary anastomoses [8,30,31,34]. There are however some limitations with this technique; it has never been validated in dogs, strict standardization is not possible and it does not allow for an actual quantification of the shunt ratio [33]. More important than a bubble score is the simple observation that dogs with severe PH did not shunt while those with only a mildly increased or normal PAP did, suggesting a role of the intrapulmonary arteriovenous anastomoses in reducing PAP.
Considering the severe histopathological changes induced by A. vasorum the reason for the absence of shunting in the present study may not have been a primary inability to open IPAVAs but that these arteriovenous anastomoses were destroyed, a possibility that also has been raised in people [31].
An interesting feature of the dogs reported here is the huge discrepancy between the severity of the disease as judged by clinical abnormalities and severity of PH on one hand, and the degree of radiographic changes on the other hand. Dog 2 had only mild pulmonary changes on thoracic radiographs but severe PH and severe and extensive pulmonary parenchymal and vascular lesions on postmortem examination. Dog 7 also had only mild pulmonary changes on the radiographic studies, but marked and diffuse changes on CT and severe PH. It may be argued that radiographs are known to be insensitive to detect pulmonary artery disease as in thromboembolism [45]. How¬ever, in the present study we have shown in dogs 2 and 3 by histopathology and in dog 7 by CT, that the most important lesions were not restricted to the vasculature. It supports the view that thoracic radiographs may be insensitive and unreliable for assessing disease severity in cases of A. vasorum, similarly to what is described in Dirofilaria immitis infection [46]. In contrast, we have pre¬viously and again in one case here shown that pulmonary CT features of dogs infected with A. vasorum very closely parallel histopathological findings [47].
Right ventricular myocardial velocities were suggestive of diastolic dysfunction (E'/A' <1) in four of five dogs, irrespective of the degree of PH.
Similarly, we had observed a change of this ratio from normal to <1 in experimentally A. vasorum infected dogs with mild PH [7]. Abnormal diastolic function as assessed by tissue Doppler imaging has been previously described to be frequent in dogs with PH, even in the face of only mild pressure overload [48]. The importance of this abnormality should not be overinterpreted, because in the present study the two dogs with mild-to-moderate PH and this pattern were already 7 and 11 years old, and E'/A' may be <1 in normal old dogs [49]. Thus, the finding was only remarkable in case 6, a young dog without documented PH. This parameter may react very sensitively to diseases affecting RV function but its true value in a clinical setting remains to be demonstrated.
Pulmonary arterial pressure is determined by pulmonary flow and vascular resistance (P = Q x R). Therefore, hyperdynamic states or RV failure may, respectively, cause an overestimation and underestimation of PAP [28,50]. In the present report the calculated PVR was increased in all dogs where it was measured, which was expected with A. vasorum—induced PH. Moreover the degree of PVR elevation was in accordance with the PH severity based on peak systolic PG, suggesting that a clinically important overestimation or under¬estimation of PH was not observed.
In dog 5 PH never reverted after treatment, even though A. vasorum was eliminated. This may have been due to a concomitant cause for the PH or irreversibility of the pulmonary vascular or parenchymal lesions induced by A. vasorum, spe¬cifically secondary pulmonary fibrosis. This latter possibility seems most likely considering the per¬sistent interstitial radiographic and CT changes in survivors [47,51]. In this dog the peripheral alveolar infiltrates resolved but a generalized severe interstitial infiltrate remained.
Important limitations of this study are those inherent to SCE as described. The absence of blood gas data or at least oxygen saturation in all dogs is another limitation. This would enable an attempt to correlate the degree of shunting with the severity of hypoxemia. However, three of five dogs with bubble score 0 were severely hypoxemic in blood gas analysis, and so the absence of shunting recruitment was unlikely to have been due to a lack of stimulus but rather due to an inability. Another important limitation is the heterogeneity of the patients. By definition, we only considered dogs with severe disease, however, there is no standard to define severe disease. Our definition was based primarily on the severity of clinical abnormalities, and the combined imaging results. Ideally, there would be a grading system probably based on CT abnormalities, quantifying the distribution and density of pulmonary changes and quantifying the extent of pulmonary parenchymal and vascular lesions. Pulmonary arterial pressure was determined non-invasively by Doppler echocardiography, which is known to be an inaccurate technique in both humans and dogs [52,53].
Several factors may hinder the accurate non-invasive measurement of PAP, such as an inadequate alignment with TR or PR jets and absence of a proper flow profile limiting correct peak velocity measurement [53]. An optimal image quality and echocardiographic technique was attempted in all dogs in order to minimize potential errors in PAP assessment. Underestimation of PAP and mis- classification of PH severity has been shown to be frequent when using echocardiography [52,53], and this may have occurred in the dogs studied here. Another potential limitation is that congenital intrapulmonary arteriovenous malformations cannot be excluded . This seems unlikely in view of the large body of evidence for the presence of normal intrapulmonary arteriovenous anastomoses in humans and dogs[2,5,6,8].
Future studies should attempt to quantify the degree of pulmonary parenchymal and vascular disease, include a larger number of dogs to determine the relationship of IPAVAs and PAP and evaluate if these anastomoses have an impact on outcome or quality of life. Finally, the role of IPAVAs should also be evaluated in other pulmo¬nary diseases.
Conclusions
Dogs with severe A. vasorum infection and docu¬mented contrast in the left heart had lower PAP than dogs with severe A. vasorum infection and absence of contrast in the left heart. Although hypothetical, this may indicate a role of IPAVAs in the regulation of PAP in dogs.
Conflicts of interest
This study was not supported by any grant or other source of direct funding.
References
- Eldridge MW. Exercise-induced intrapulmonary arterio¬venous shunting in healthy humans. J Appl Physiol 2004; 97:797-805.
- Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med 2007;176:300-305.
- Lovering AT, Eldridge MW, Stickland MK. Counterpoint: exercise-induced intrapulmonary shunting is real. J Appl Physiol 2009;107:994-997.
- Gudavalli A, Kalaria VG, Chen X, Schwarz KQ. Intra¬pulmonary arteriovenous shunt: diagnosis by saline con¬trast bubbles in the pulmonary veins. J Am Soc Echocardiogr 2002;15:1012-1014.
- Tobin CE. Arteriovenous shunts in the peripheral pulmo¬nary circulation in the human lung. Thorax 1966;21: 197-204.
- Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-micron arteriovenous path¬ways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol 2007;292:H1777-H1781.
- Matos JM, Schnyder M, Bektas R, Makara M, Kutter A, Jenni S, Deplazes P, Glaus T. Recruitment of arterio¬venous pulmonary shunts may attenuate the develop¬ment of pulmonary hypertension in dogs experimentally infected with Angiostrongylus vasorum. J Vet Cardiol 2012;14:313-322.
- Laurie SS, Yang X, Elliott JE, Beasley KM, Lovering AT. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol 2010;109: 1072-1079.
- Lovering AT, Elliott JE, Beasley KM, Laurie SS. Pulmonary pathways and mechanisms regulating transpulmonary shunting into the general circulation: an update. Injury 2010;41(Suppl 2):S16-S23.
- Gossage JR, Kanj G. Pulmonary arteriovenous malforma¬tions. A state of the art review. Am J Respir Crit Care Med 1998;158:643-661.
- Jenni SD, Makara MA, Jenni R, Ohlerth S, Glaus TM. Diagnosis of pulmonary arterio-venous fistula and persis¬tent left cranial vena cava by 3-dimensional computed tomographic reconstruction. J Vet Intern Med 2009;23: 190-195.
- Esteves I, Tessier D, Dandrieux J, Polack B, Carlos C, Boulanger V, Muller C, Pouchelon JL, Chetboul V. Rever¬sible pulmonary hypertension presenting simultaneously with an atrial septal defect and angiostrongylosis in a dog. J Small Anim Pract 2004;45:206-209.
- Nicolle AP, Chetboul V, Tessier-Vetzel D, Carlos Sampedrano C, Aletti E, Pouchelon J-L. Severe pulmonary arterial hypertension due to Angiostrongylosus vasorum in a dog. Can Vet J Rev Vet Can 2006;47:792-795.
- Traversa D, Torbidone A, Malatesta D, Guglielmini C. Occurrence of fatal canine Angiostrongylus vasorum infection in Italy. Vet Parasitol 2008;152:162-166.
- Glaus T, Schnyder M, Dennler M, Tschuor F, Wenger M, Sieber-Ruckstuhl N. Natural infection with Angiostrongylus vasorum: characterisation of 3 dogs with pulmonary hypertension. Schweiz Arch Fur Tierheilkd 2010;152: 331 -338.
- Swann JW, Sudunagunta S, Covey HL, English K, Hendricks A, Connolly DJ. Evaluation of red cell dis¬tribution width in dogs with pulmonary hypertension. J Vet Cardiol 2014;16:227-235.
- Borgeat K, Sudunagunta S, Kaye B, Stern J, Luis Fuentes V, Connolly DJ. Retrospective evaluation of moderate-to- severe pulmonary hypertension in dogs naturally infec¬ted with Angiostrongylus vasorum. J Small Anim Pract 2015;56:196-202.
- Prestwood A, Greene C, Mahaffey E, Burgess D. Experimental canine angiostrongylosis: I. Pathologic manifestations. J Am Vet Med Assoc 1981;17:491-497.
- Caruso JP, Prestwood AK. Immunopathogenesis of canine angistrongylosis: pulmonary effects of infection. Comp Immunol Microbiol Infect Dis 1988;11:85-92.
- Kranjc A, Schnyder M, Dennler M, Fahrion A, Makara M, Ossent P, Morgan J, Deplazes P, Glaus TM. Pulmonary artery thrombosis in experimental Angiostrongylus vasorum infection does not result in pulmonary hypertension and echocardiographic right ventricular changes: echocardiography in experimental Angiostrongylosis. J Vet Intern Med 2010;24:855-862.
- Schnyder M, Fahrion A, Riond B, Ossent P, Webster P, Kranjc A, Glaus T, Deplazes P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol Res 2010;107: 1471-1480.
- Glaus T. Pulmonary (arterial) hypertension. In: Cote E, editor. Clinical veterinary advisory: dogs and cats. St Louis, MO: Elsevier; 2015. p. 861-863.
- Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, Moses BL. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247-252.
- Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992-1996. J Vet Intern Med 1999;13:440-447.
- Martin-Duran R, Larman M, Trugeda A, Vazquez de Prada JA, Ruano J, Torres A, Figueroa A, Pajaron A, Nistal F. Comparison of Doppler-determined elevated pulmonary arterial pressure with pressure measured at cardiac catheterization. Am J Cardiol 1986;57:859-863.
- Pariaut R, Saelinger C, Strickland KN, Beaufrere H, Reynolds CA, Vila J. Tricuspid annular plane systolic excursion (TAPSE) in dogs: reference values and impact of pulmonary hypertension. J Vet Intern Med 2012;26: 1148-1154.
- Opotowsky AR, Clair M, Afilalo J, Landzberg MJ, Waxman AB, Moko L, Maron BA, Vaidya A, Forfia PR. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol 2013;112:873-882.
- Boon JA. Hypertensive heart disease. In: Boon JA, editor. Veterinary echocardiography. Chichester, UK: Wiley- Blackwell; 2011. p. 335-351.
- Elliott JE, Nigam SM, Laurie SS, Beasley KM, Goodman RD, Hawn JA, Gladstone IM, Chesnutt MS, Lovering AT. Prev¬alence of left heart contrast in healthy, young, asympto¬matic humans at rest breathing room air. Respir Physiol Neurobiol 2013;188:71-78.
- Lovering AT, Stickland MK, Amann M, Murphy JC, O'Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia pre¬vents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol 2008;586:4559-4565.
- Cameron Norris H, Mangum TS, Duke JW, Straley TB, Hawn JA, Goodman RD, Lovering AT. Exercise- and hypoxia-induced blood flow through intrapulmonary arteriovenous anastomoses is reduced in older adults. J Appl Physiol 2014;116:1324-1333.
- Tremblay JC, Lovering AT, Ainslie PN, Stembridge M, Burgess KR, Bakker A, Donnelly J, Lucas SJE, Lewis NCS, Dominelli PB, Henderson WR, Dominelli GS, Sheel AW, Foster GE. Hypoxia, not pulmonary vascular pressure, induces blood flow through intrapulmonary arteriovenous anastomoses: hypoxia induces blood flow through IPAVA. J Physiol 2015;593:723-737.
- Laurie SS, Elliott JE, Goodman RD, Lovering AT. Cat¬echolamine-induced opening of intrapulmonary arterio¬venous anastomoses in healthy humans at rest. J Appl Physiol 2012;113:1213-1222.
- La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt J-U, Heidbuchel H, Prior DL. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 2010;109:1307-1317.
- Le Roux BT, Gibb BH, Wainwright J. Pulmonary arterio¬venous fistula with bilharzial pulmonary hypertension. Br Heart J 1970;32:571-574.
- Hasan A, Sastry BKS, Aleem MA, Reddy G, Mahmood S. Regression of pulmonary artery hypertension due to development of a pulmonary arteriovenous malformation. Indian Heart J 2014;66:535-538.
- Niden AH, Aviado DM. Effects of pulmonary embolism on the pulmonary circulation with special reference to arteriovenous shunts in the lung. Circ Res 1956;4:67-73.
- Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev 2006;34:99-106.
- Galambos C, Sims-Lucas S, Abman SH. Histologic evidence of intrapulmonary anastomoses by three-dimensional reconstruction in severe bronchopulmonary dysplasia. Ann Am Thorac Soc 2013;10:474-481.
- Acker SN, Mandell EW, Sims-Lucas S, Gien J, Abman SH, Galambos C. Histologic identification of prominent intrapulmonary anastomotic vessels in severe congenital dia¬phragmatic hernia. J Pediatr 2015;166:178-183.
- Bryan TL, van Diepen S, Bhutani M, Shanks M, Welsh RC, Stickland MK. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol 2012;113:541-548.
- Elliott JE, Duke JW, Hawn JA, Halliwill JR, Lovering AT. Increased cardiac output, not pulmonary artery systolic pressure, increases intrapulmonary shunt in healthy humans breathing room air and 40% O2: blood flow through IPAVAs impairs pulmonary gas exchange efficiency. J Physiol 2014;592:4537-4553.
- Prinzmetal M, Ornitz M, Simkin B, Bergman H. Arterio¬venous anastomoses in liver, spleen and lungs. Am J Physiol 1948;152:48-52.
- Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol 2008;104: 1418-1425.
- Fluckiger MA, Gomez JA. Radiographic findings in dogs with spontaneous pulmonary thrombosis or embolism. Vet Radiol 1984;25:124-131.
- Nelson T, McCall JW, Carithers D. Current canine guide¬lines for the prevention, diagnosis, and management of heartworm (Dirofilaria immitis) infection in dogs. Revised. American Heartworm Society; July 2014.
- Dennler M, Makara M, Kranjc A, Schnyder M, Ossent P, Deplazes P, Ohlerth S, Glaus TM. Thoracic computed tomography findings in dogs experimentally infected with Angiostrongylus vasorum. Vet Radiol Ultrasound 2011;52: 289-294.
- Serres F, Chetboul V, Gouni V, Tissier R, Sampedrano CC, Pouchelon J-L. Diagnostic value of echo-Doppler and tis¬sue Doppler imaging in dogs with pulmonary arterial hypertension. J Vet Intern Med 2007;21:1280-1289.
- Mercier E, Mathieu M, Sandersen CF, Delvaux FH, Clercx CM, Mc Entee K. Evaluation of the influence of age on pulmonary arterial pressure by use of right ventricular catheterization, pulsed-wave Doppler echocardiography, and pulsed-wave tissue Doppler imaging in healthy Bea¬gles. Am J Vet Res 2010;71:891-897.
- Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 2003;41:1021-1027.
- Boag AK, Lamb CR, Chapman PS, Boswood A. Radiographic findings in 16 dogs infected with Angiostrongylus vasorum. Vet Rec 2004;154:426-430.
- Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accu¬racy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615-621.
- Soydan LC, Kellihan HB, Bates ML, Stepien RL, Consigny DW, Bellofiore A, Francois CJ, Chesler NC. Accuracy of Doppler echocardiographic estimates of pul¬monary artery pressures in a canine model of pulmonary hypertension. J Vet Cardiol 2015;17:13-24.
- Cornell CC, Kittleson MD, Torre PD, Huaggstroum J, Lombard CW, Pedersen HD, Vollmar A, Wey A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311-321.
- Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in West Highland White Terriers with chronic pulmonary disease. J Vet Intern Med 2006;20:912-920.
- Baumwart RD, Meurs KM, Bonagura JD. Tei index of myocardial performance applied to the right ventricle in normal dogs. J Vet Intern Med 2005;19:828-832.
- Visser LC, Scansen BA, Schober KE, Bonagura JD. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: repeatability and reference intervals. J Vet Cardiol 2014.
^Наверх









