Association of the myosin binding protein C3 mutation (MYBPC3 R820W) with cardiac death in a survey of 236 Ragdoll cats

Author information
Borgeat K., Casamian-Sorrosal D., Helps C., Luis Fuentes V., Connolly D.J. Association of the myosin binding protein C3 mutation (MYBPC3 R820W) with cardiac death in a survey of 236 Ragdoll cats // J Vet Cardiol. 2014 Jun;16(2):73-80.
Abstract
OBJECTIVES: A mutation identified in the myosin binding protein C3 gene (MYBPC3 R820W) has been associated with hypertrophic cardiomyopathy (HCM) in Ragdoll cats. Ragdolls with HCM are reported to have a poor prognosis and homozygous cats seem particularly likely to develop severe HCM, although the outcome in Ragdolls tested for the MYBPC3 mutation has not been reported. We aimed to determine the influence of genotype on survival in Ragdoll cats using a questionnaire, and hypothesized that homozygous Ragdolls had shorter lifespans and were more likely to suffer cardiac death than heterozygous or wild-type (WT) cats.
ANIMALS: 251 client owned Ragdoll cats.
METHODS: A questionnaire for breeders/owners of MYBPC3 genotyped Ragdolls included items related to genotype, age, sex, current status (alive/dead), and date and circumstances of death. Death was categorized as cardiac or non-cardiac. Survival was analyzed using Kaplan-Meier curves and log rank tests.
RESULTS: Completed questionnaires were received for 236 cats (156 WT, 68 heterozygous, 12 homozygous). Median survival time for homozygous cats was 5.65 years (95%CI 0.4-10.9 years) compared to heterozygous (>16.7 years) or WT (>15.2 years). Homozygous cats were more likely to die from cardiac death (p = 0.004 vs. WT; p = 0.003 vs. heterozygous) and had significantly shorter time to cardiac death (vs. WT p < 0.001; vs. heterozygous p < 0.001).
CONCLUSIONS: Ragdoll cats homozygous for the MYBPC3 R820W mutation have a shorter survival time than WT or heterozygous cats. This suggests a mode of inheritance that follows an incomplete dominance pattern.
Abbreviations
- 95%CI 95% confidence intervals
- HCM hypertrophic cardiomyopathy
- MST median survival time
- MYBPC3 myosin binding protein C3
- UK United Kingdom
- USA United States of America
- WT wild type (genotype)
Introduction
Hypertrophic cardiomyopathy (HCM) is a common cardiac disease in humans, affecting approximately 1:500 people.1 It is well identified as a heritable disease and has a complex genetic etiology.2 Approximately 50% of these patients have a known sarcomeric mutation.3,4 Others are presumed to be affected by either a spontaneously occurring, de novo mutation or a previously unidentified HCM-associated mutation.2
Hypertrophic cardiomyopathy-associated mutation carriers within the same family might have different phenotypic expression of heart disease, and it is thought that environmental factors, modifier genes, and the presence of multiple sarcomeric mutations are important in the phenotypic expression of disease.1,2,5—13 Long-term outcome of patients with HCM is worse for carriers of HCM- associated mutations compared to those who test negative for known mutations.14 The inheritance pattern of HCM-associated sarcomeric mutations in humans is largely accepted to be autosomal dominant with an age dependent penetrance.2,15,16
Ragdoll cats are over-represented in studies of feline HCM, suggesting a predisposition to the disease.17,c Hypertrophic cardiomyopathy in this breed has been reported to be familial, with an early onset of clinical signs and a poor prognosis.12 A substitution mutation in the myosin binding protein C3 gene (MYBPC3 R820W) is associated with HCM in Ragdoll cats and leads to alteration of the amino acid sequence and disruption of a conserved region of MYBPC3.18 Within screened populations of cats, this mutation has a reported prevalence of 17—30.1%.19
In humans, numerous mutations of the MYBPC3 gene are associated with development of an HCM phenotype, suggesting that this gene is important in the pathogenesis of the disease.20—23 An identical substitution mutation to that detected in Ragdolls (MYBPC3 R820W) has been identified in a human family affected by HCM,24 wherein homozygous individuals have a more severe clinical expression than closely related heterozygotes that demonstrate a mild or late-onset phenotype. Also, a genetic mutation at a different locus of the MYBPC3 gene has been associated with HCM in Maine Coon cats.25 Recent research suggests that homozygous Maine Coons are more likely to develop an echocardiographic Hypertrophic cardiomyopathy phenotype than heterozygous or wild- type (WT) cats.19 Currently, there are no published studies that report the outcome of a large cohort of Ragdoll cats tested for the MYBPC3 R820W mutation.
This study aimed to determine the survival time and cause of death for a large cohort of Ragdolls tested for the MYBPC3 R820W mutation. We hypothesized that homozygous cats have a shorter lifespan and are more likely to suffer cardiac death than heterozygous or WT cats.
Animals, materials and methods
Data were obtained by means of a questionnaire targeting owners or breeders of Ragdoll cats that had been tested for the MYBPC3 R820W mutation at commercial laboratories. Each questionnaire provided details from a unique cat; respondents owning multiple cats completed a separate questionnaire for each animal. Questions focused on 4 main areas of interest: signalment (age/sex/neu- ter status), genotype (WT/heterozygous/homozygous), current status (alive/dead) and date and circumstances of death if applicable. Also included were questions about dietary history, current health status, ongoing illnesses, medications (including cardiac) and the reasons for any recent veterinary visits. These factors were not designed to be analyzed as part of this study, but were included to encourage completion of the questionnaire. Respondents were given the opportunity to provide their contact details and give consent for investigators to contact them directly if necessary. Questionnaire design was discussed with a veterinary epidemiologist and refined after it was tested for clarity by cat owners amongst nonclinical hospital staff.
The final questionnaire was converted to an online format using a web-based survey tool.e A study specific websitef was created using a free online tool8 and linked to the questionnaire. The questionnaire was available to complete online for one year from 04/30/2012. The genotype and current status (alive/dead) were compulsory questions at the time of questionnaire launch. Date of birth became compulsory after the questionnaire had been open for approximately 4 months. The study website was publicized online via social media, through the British Ragdoll Club [the largest Ragdoll breed club in the United Kingdom (UK)] and talks at pedigree cat shows. In addition to the online format, paper questionnaires were available if respondents requested this by email. These were also distributed in person at cat shows (150 copies).
A study-specific email address was available for respondents to contact the investigators. This could be used to update information during the study period if the status of a patient changed (e.g. if acat died after a questionnaire had been completed).
Statistical analysis
At the end of the inclusion period (04/30/2013), data collected from the questionnaire were exported into a spreadsheet^ Circumstances of death were classified as cardiac or non-cardiac. Cardiac death was defined as any of the following: sudden and unexpected death; death or euthanasia preceded by an unexplained episode of respiratory distress or the sudden loss of use of one or more limbs; or euthanasia due to sus- pected/known arterial thromboembolism, worsening clinical signs of congestive heart failure, or cardiogenic shock. Cats were excluded if the date of birth or circumstances of death were unknown or incomplete.
The database was reformatted for statistical analysis and copied to a statistical software program/ A Chi squared test was performed to investigate associations between genotype and age group, and genotype and cardiac death. For survival analysis, T0 was considered to be the date of birth so "survival” was synonymous with "lifespan”. Kaplan—Meier survival curves were generated and a Log rank test performed to identify differences in survival between cats of different genotypes and sex (cardiac vs. non-cardiac death, and all-cause mortality).
Cats alive at the time of questionnaire completion or those that suffered a non-cardiac death were right-censored. Statistical significance was set at p < 0.05. Where multiple comparisons were carried out between age groups, Bonferroni correction was applied (corrected level of significance p < 0.008). Numerical data were expressed as median (range).
Results
Data were available for 251 cats in total, comprising 215 online questionnaires and 36 paper questionnaires. The majority of cats were female (163/251, 64.9%) and a minority were neutered (42/251, 16.7%). Information on gender was not completed for 5 cats.
Overall, 110 individual respondents completed the questionnaire. Although difficult to define a true response rate, 404 unique computers/devices accessed the website during the study period. We estimated that the response rate was 27.2% (110 respondents/404 visitors). The questionnaire was completed by respondents worldwide: UK (62.9%), mainland Europe (17%), United States of America (USA)/ Canada (14.3%) and Australia/New Zealand (5.3%). Genotyping had been performed in laboratories from corresponding locations: UK (74.9%), mainland Europe (7.2%), USA/Canada (7.2%) and Australia/New Zealand (8.8%).
Of 251 cats, 15 were excluded from survival analysis, because either no date of birth was provided (13/15) or the mutation status (heterozygous vs. homozygous) was unknown (2/15). After exclusions, data on 236 cats were suitable for survival analysis. Of these cats, 66.1% were WT (156/236), 28.8% were heterozygous (68/236) and 5.1% were homozygous (12/236). Overall mutation prevalence in this population was 33.9%. Median age of cats at the time of last contact (date of questionnaire completion or date of death) was 5.15 years (0.14—16.74 years), suggesting a bias towards younger cats (Fig. 1). Genotype was not evenly distributed across age groups (p = 0.003), with a greater proportion of heterozygous cats in the oldest age quartile (>8.21 years) compared to the youngest age quartile (<2.33 years, p < 0.001) There was no significant difference identified by pairwise comparison of the other age quartiles (p > 0.008; Fig. 2).
The overall event rate in the population was low, with 30/236 (12.7%) cats dead at the time of questionnaire completion. Circumstances of death were classified as cardiac in 15/30 and non-cardiac in 15/30 cats. Non-cardiac causes consisted of euthanasia due to chronic illness of unknown cause (7/15 cats), confirmed neoplasia (5/15 cats), death due to motor vehicle accident (1/15 cats), acute coughing episode (1/15 cats) and unknown (1/15 cats). Cardiac causes consisted of sudden and unexpected death (7/15 cats), death after acute and unexplained respiratory distress (2/15 cats), sudden death with HCM confirmed on postmortem (1/15 cats), euthanasia due to refractory clinical signs of congestive heart failure (3/15 cats) and euthanasia due to clinical signs of arterial thromboembolism (2/15 cats).
Age Group
Figure 1 Histogram showing the distribution of age at last contact (date of questionnaire completion or death) for Ragdolls reported in the questionnaire. The population was skewed towards younger cats.
Figure 2 Bar chart showing the number of cats of each genotype in different age quartiles.
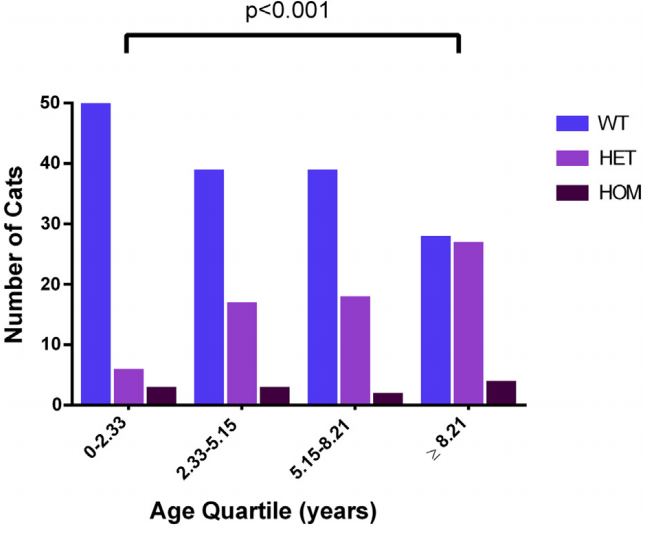
Chi squared tests were used to perform pairwise comparisons between quartiles. A Bonferroni correction for multiple comparisons was applied (significance p < 0.008). HET, heterozygous; HOM, homozygous; WT, wild type.
The median survival time (MST) for all cats was 14.1 years (95%CI 12.8—15.4 years). Analysis of cardiac death according to genotype showed a significantly shorter time to cardiac death for homozygous cats (MST 5.65 years, 95%CI 0.4—10.9 years) than for heterozygous (p < 0.001) or WT (p < 0.001) cats (Table 1). There was no significant difference in survival time between heterozygous and WT cats (p = 0.848, Fig. 3). Analysis of allcause mortality showed a similar pattern, with homozygous cats dying at a younger age than heterozygous (p < 0.001) or WT (p < 0.001) cats. There was no significant difference in survival between heterozygous and WT cats (p = 0.587). There was no influence of sex on either cardiac or all-cause mortality. In those cats reported to have died (n = 30), cardiac death was significantly more likely in homozygous cats than WT (p = 0.004) or heterozygous (p = 0.003) cats. There was no significant difference in the proportion of cats suffering a cardiac death between the WT and heterozygous groups. No homozygous cats died a non-cardiac death, but 4 WT cats were reported to have died as a result of cardiac causes.
Discussion
The results of this questionnaire-based study suggest that Ragdoll cats homozygous for the MYBPC3 R820W mutation die at a younger age than heterozygous or WT cats, and have shorter survival times to cardiac death. Despite their shorter MST, some homozygous cats were reported to have lived longer than 10 years. We did not identify a significant difference in outcome between heterozygous and WT individuals. If homozygous cats are more likely to develop HCM at a younger age and have progressive disease with a poorer prognosis than other genotypes, the phenotypic expression of the MYBPC3 R820W mutation in Ragdolls would seem to follow the same pattern as that identified in humans.24 We have not tested this hypothesis and prospective, longitudinal, echocardiographic studies are necessary to investigate this further. Homozygous Maine Coon cats have a much greater risk of developing an HCM phenotype identified by echocardiography than heterozygous or WT cats (hazard ratio 26.4).19
From our data, we were unable to determine whether or not heterozygous cats are more likely to develop HCM than WT cats. One might hypothesize that if HCM does develop more frequently in heterozygous cats, it has a more benign phenotype than in homozygous cats, since there was no significant difference in the age of death between heterozygous and WT cats in this study. However, the population was relatively young, which limits the usefulness of determining the prevalence of a disease with an age-dependent penetrance, such as HCM.2 Despite the study limitations of a younger study group, an overall low event rate, and a relatively small percent of homozygous cats, a shorter time to cardiac death was clearly identified for the homozygous genotype in the current study.
Longeri and colleagues19 showed in a prospectively screened population that heterozygous Maine Coons were more likely to develop an echocardiographic HCM phenotype than WT cats, but outcome data were not reported. Expression of a genetic disease might be affected by the phenomenon of incomplete dominance, where heterozygous individuals have a different phenotypic expression than homozygous individuals, with the former often being of intermediate appearance.26 The results of our questionnaire analysis suggest that incomplete dominance might be important in the development of HCM in Ragdoll cats with the MYBPC3 R820W mutation. In humans, two HCM- associated MYBPC3 mutations (R820W is one) follow a similar pattern.24,27
This is important information to consider when counselling Ragdoll breeders, breed clubs and owners regarding the interpretation of genetic testing. Although removing potential breeding animals can lead to unanticipated consequences, it is also possible that if Ragdoll breeders avoid the breeding of two heterozygous cats, the resultant lack of homozygous offspring might eventually reduce the frequency and severity with which HCM is expressed in this breed. Interestingly, our questionnaire identified a greater proportion of heterozygous cats in the oldest age quartile compared to the youngest. This was supported by an increasing visual trend (Fig. 2). From our data it is impossible to explain this finding. One possibility is that selective breeding since the time that the MYBPC3 genetic test became commercially available has led to fewer heterozygous cats being produced in recent years. Although this seems a reasonable explanation, further studies would be needed to confirm this possibility.
The estimated response rate to this questionnaire was 27.2%. For paper copies, the response rate was even lower, but many respondents were directed to the website by the paper copy, from where it was easier to complete data on multiple cats. The online response rate was difficult to calculate accurately, but we used the total number of unique website visits, tallied by the website host based on each unique computer/ device identity that viewed the site. This means that one respondent would be counted as two views if they accessed the website from a mobile internet browser and then decided to complete the questionnaire on a home computer. Therefore our reported response rate is a conservative estimate and might not represent the true response rate.
Figure 3 Kaplan Meier survival curve showing the difference in survival time (lifespan) to cardiac death for Ragdoll cats of different MYBPC3 R820W genotype (Logrank p < 0.001).

Median survival time for homozygous cats was 5.65 years (95%CI 0.4—10.9 years), significantly shorter than for heterozygous (>16.7 years, p < 0.001) or WT (>15.2 years, p < 0.001) cats. There was no significant difference in survival time between heterozygous and WT cats (p = 0.848). HET, heterozygous; HOM, homozygous; WT, wild type.
Like many studies investigating disease in pedigree cats, this population is likely to be biased towards breeding cats. This is supported by the female sex bias and the large proportion of non- neutered cats. Website data and laboratory submission responses identified a bias towards cats in the UK. Too few cats were reported from different geographical areas to justify sub-analysis by region, but it might be the case that prevalence of the MYBPC3 R820W mutation varies by region. This could be explained by genetic differences in breeding families, different breeding policies or variable protocols on breeding with MYBPC3 positive cats.
The overall prevalence of the MYBPC3 R820W mutation in this study was higher than reported from commercial laboratories in Italy (17%) and the USA (22.9%),19 but similar to that reported from the UK (30.1%).d Although it is conceivable that the higher prevalence of the MYBPC3 mutation reported in this study was caused by a recall bias in questionnaire respondents, our data was biased towards the UK and might reflect that the MYBPC3 mutation is more prevalent in this country than others.
Other limitations of this study are typical for questionnaire-based research. The responses submitted might not be representative of the entire Ragdoll population. Recall bias is a possible limitation of any questionnaire-based study, where owners/breeders that have had distressing experiences with feline heart disease are more likely to respond to a questionnaire on the subject. Recall bias was unfortunately impossible to quantify accurately in this study. The study was reliant on data provided by breeders/owners, so clinical data were lacking and no echocardiographic studies were performed. Results of genotyping could not be verified in this study. However, Ragdoll breeders in the UK are encouraged by breed clubs to keep original documentation related to genetic testing. This reduces the risk of inaccurate recall by respondents to this questionnaire. Ideally, results would have been confirmed by cross- referencing questionnaire responses to databases held by commercial laboratories. However, this was impossible due to personal data protection legislation. Despite clinical data such as the date of diagnosis of HCM, clinical signs and medical treatments prescribed being reported in the questionnaire, the responses received suggested this information was too unreliable for statistical analysis and thus it was not included in this study. We decided to focus our analysis on the most reliable end-point; death.
The overall event rate was low, as was the number of homozygous cats. Although these limitations were likely to reduce the chance of identifying a significant difference between groups, a highly significant difference in survival was identified. It therefore seems likely that the shorter lifespan of homozygous cats identified represents a true difference. It is possible that the sample size and age-distribution of heterozygous and WT cats was not sufficient to detect a more subtle difference in time to cardiac death between these groups.
Conclusions
Ragdoll cats homozygous for the MYBPC3 R820W mutation had a significantly shorter time to cardiac death and died at a younger age than heterozygous or WT cats in this study. There was no significant difference in survival to cardiac death or all-cause mortality between heterozygous and WT cats. This suggests that the MYBPC3 R820W mutation has an incomplete dominance pattern of inheritance in Ragdoll cats. Considering the worse prognosis of Ragdoll cats with a homozygous genotype is important when advising on Ragdoll breeding policy. Further prospective, longitudinal study of heterozygous and WT cats will be necessary to determine whether the MYBPC3 mutation affects the phenotypic expression of HCM and the incidence of cardiac death in these individuals.
Conflict of interest
This study was presented as an abstract at the American College of Veterinary Internal Medicine Forum 2013, Seattle, USA. The authors have no conflict of interest to declare with regard to this study.
Acknowledgements
The authors gratefully acknowledge the British Ragdoll Cat Club for their assistance in promoting the questionnaire and Jessica Dincer for her assistance in collecting data.
References
- Maron BJ. Hypertrophic cardiomyopathy - a systematic review. J Am Med Assoc 2002;287:1308-1320.
- Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years clinical perspectives. J Am Coll Cardiol 2012;60:705-715.
- Erdmann J, Daehmlow S, Wischke S, Senyuva M, Werner U, Raible J, Tanis N, Dyachenko S, Hummel M, Hetzer R, Regitz-Zagrosek V. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin Genet 2003;64:339-349.
- Andersen PS, Havndrup O, Hougs L, Sorensen KM, Jensen M, Larsen LA, Hedley P, Thomsen AR, Moolman-Smook J, Christiansen M, Bundgaard H. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Hum Mutat 2009;30: 363-370.
- Alpert NR, Mohiddin SA, Tripodi D, Jacobson-Hatzell J, Vaughn-Whitley K, Brosseau C, Warshaw DM, Fananapazir L. Molecular and phenotypic effects of heterozygous, homozygous, and compound heterozygote myosin heavy-chain mutations. Am J Physiol - Heart C 2005;288:H1097-H1102.
- Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hypertrophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet 2005;42:e59.
- Kelly M, Semsarian C. Multiple mutations in genetic cardiovascular disease: a marker of disease severity? Circ Cardiovasc Genet 2009;2:182-190.
- Patel R, Lim DS, Reddy D, Nagueh SF, Lutucuta S, Sole MJ, Zoghbi WA, Quinones MA, Roberts R, Marian AJ. Variants of trophic factors and expression of cardiac hypertrophy in patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol 2000;32:2369-2377.
- Brugada R, Kelsey W, Lechin M, Zhao G, Yu QT, Zoghbi W, Quinones M, Elstein E, Omran A, Rakowski H, Wigle D, Liew CC, Sole M, Roberts R, Marian AJ. Role of candidate modifier genes on the phenotypic expression of hypertrophy in patients with hypertrophic cardiomyopathy. J Invest Med 1997;45:542-551.
- Maron BJ, McKenna WJ, Danielson GK, Kappenberger JKB, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, Spirito P, Cate FJT, Wigle ED. American college of cardiology/euro- pean society of cardiology clinical expert consensus document on hypertrophic cardiomyopathy - a report of the American college of cardiology foundation task force on clinical expert consensus documents and the European society of cardiology committee for practice guidelines. Eur Heart J 2003;24:1965-1991.
- Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, Biller L, Lesser JR, Udelson JE, Manning WJ, Appelbaum E. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009;54:220-228.
- Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40-47.
- Ciro E, Nichols 3rd PF, Maron BJ. Heterogeneous morphologic expression of genetically transmitted hypertrophic cardiomyopathy. Two-dimensional echocardiographic analysis. Circulation 1983;67:1227-1233.
- Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc 2008;83: 630-638.
- Ho CY. Genetic considerations in hypertrophic cardiomyopathy. Prog Cardiovasc Dis 2012;54:456-460.
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies - an american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 2006;113:1807-1816.
- Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010;51:540-547.
- Meurs KM, Norgard MM, Ederer MM, Hendrix KP, Kittleson MD. A substitution mutation in the myosin binding protein c gene in ragdoll hypertrophic cardiomyopathy. Genomics 2007;90:261-264.
- Longeri M, Ferrari P, Knafelz P, Mezzelani A, Marabotti A, Milanesi L, Pertica G, Polli M, Brambilla PG, Kittleson M, Lyons LA, Porciello F. Myosin-binding protein c DNA variants in domestic cats (a31p, a74t, r820w) and their association with hypertrophic cardiomyopathy. J Vet Intern Med 2013; 27:275-285.
- Erdmann J, Raible J, Maki-Abadi J, Hummel M, Hammann J, Wollnik B, Frantz E, Fleck E, Hetzer R, Regitz-Zagrosek V. Spectrum of clinical phenotypes and gene variants in cardiac myosin-binding protein c mutation carriers with hypertrophic cardiomyopathy. J Am Coll Cardiol 2001;38: 322-330.
- Niimura H, Bachinski LL, Sangwatanaroj S, Watkins H, Chudley AE, McKenma W, Kristinsson A, Roberts R, Sole M, Maron BJ, Seidman JG, Seidman CE. Mutations in the gene for cardiac myosin-binding protein c and late-onset familial hypertrophic cardiomyopathy. New Engl J Med 1998;338: 1248-1257.
- Harris SP, Lyons RG, Bezold KL. In the thick of it: Hcm- causing mutations in myosin binding proteins of the thick filament. Circ Res 2011;108:751-764.
- Andersen PS, Havndrup O, Bundgaard H, Larsen LA, Vuust J, Pedersen AK, Kjeldsen K, Christiansen M. Genetic and phenotypic characterization of mutations in myosin-binding protein c (mybpc3) in 81 families with familial hypertrophic cardiomyopathy: total or partial haploinsufficiency. Eur J Hum Genet 2004;12:673-677.
- Ripoll Vera T, Monserrat Iglesias L, Hermida Prieto M, Ortiz M, Rodriguez Garcia I, Govea Callizo N, Gomez Navarro C, Rosell Andreo J, Gamez Martinez JM, Pons Llado G, Cremer Luengos D, Torres Marques J. The r820w mutation in the mybpc3 gene, associated with hypertrophic cardiomyopathy in cats, causes hypertrophic cardiomyopathy and left ventricular non-compaction in humans. Int J Cardiol 2010;145:405-407.
- Meurs KM, Sanchez X, David RM, Bowles NE, Towbin JA, Reiser PJ, Kittleson JA, Munro MJ, Dryburgh K, Macdonald KA, Kittleson MD. A cardiac myosin binding protein c mutation in the maine coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet 2005;14: 3587-3593.
- Nicholas F. Chapter 7, is it inherited? In: Nicholas F, editor. Introduction to veterinary genetics. United Kingdom, Wiley- Blackwell; 2009. p. 150-157.
- Zahka K, Kalidas K, Simpson MA, Cross H, Keller BB, Galambos C, Gurtz K, Patton MA, Crosby AH. Homozygous mutation of mybpc3 associated with severe infantile hypertrophic cardiomyopathy at high frequency among the amish. Heart 2008;94:1326-1330.
^Наверх









