Mitral valve repair in dogs

Author information
Uechi M1. Mitral valve repair in dogs // J Vet Cardiol. 2012 Mar;14(1):185-92.
Abstract
Prognosis for dogs with severe mitral regurgitation is poor with medical therapy alone. Open surgical mitral valve repair consisting of circumferential mitral annuloplasty and artificial chordal replacement confers durability and improved long-term clinical outcome without a need for long-term antithrombotic therapies. This approach has been successfully used in canine patients, including small-breed dogs. Methods for mitral valve repair applicable to small dogs are described.
Abbreviations
CPB cardiopulmonary bypass
ePTFE expanded-polytetrafluoroethylene
Introduction
Dogs with severe mitral regurgitation have a poor prognosis despite recent medical advances for the management of heart failure.1 While pimobendan improves the clinical condition and prognosis in affected dogs, 80% of these patients are likely to worsen or die within 2 years of diagnosis.2 Because medical management of secondary heart failure is incapable of correcting disorders of the mitral valve complex, surgical intervention is required to improve clinical condition and prognosis. Surgical treatments for mitral regurgitation include valve replacement and valve repair. While these are standard therapies in humans,3-6 mitral valve surgery has been reported in comparatively few veterinary patients.7-18 Open heart surgery is more commonly attempted in larger breeds, as it is difficult to perform cardiopulmonary bypass (CPB) in small dogs using traditional methods.14 Recent improvements in CPB techniques have facilitated its effective use in small-breed dogs18-20 and even in a cat.21 These advances have enabled successful mitral valve surgery in a wider range of dog sizes.
Faulty mitral valves can be replaced with a mechanical valve16 or a bioprosthetic valve.10,12,17 In the case of prosthetic valves, long¬term survival is promoted by carefully matching the size of a prosthetic heart valve to the dog and strong efforts to avoid thrombosis.16 One advan¬tage of bioprosthetic valves is that they are less thrombogenic than mechanical valves.10,12,17,22 Bioprosthetic valve replacement may be particu¬larly useful in cases of mitral valve dysplasia, in which the original mitral valve is difficult to repair.10,12,17 One potential drawback of bio¬prosthetic valves is their tendency to calcify or degenerate over time, thus reducing long-term survival.23 Dogs receiving bioprosthetic valves have been known to survive >17 months,10,12,17 with no reports to date of calcification bioprosthetic heart valves in dogs.
In both human4,5 and veterinary medi- cine,13,14,18 properly performed mitral valve repair, including both circumferential mitral annuloplasty and artificial chordal replacement, confers excellent durability and improved longterm clinical outcomes without the need for long-term antithrombotic therapy. Thus, mitral valve repair may become an important treatment for mitral regurgitation in dogs. This report describes methods for mitral valve repair in dogs . Cardiopulmonary bypass Mitral valve surgery requires stopping the heart and support of cardiac and pulmonary function by CPB. This is accomplished by a heart-lung machine with an extracorporeal circuit, oxygenator, and heat exchanger.
The CPB circuit is filled with a priming solution consisting of 20% D- mannitol, 7% sodium bicarbonate, and heparin sodium in acetate Ringer’s solution. In dogs weighing <4 kg, priming solution should be replaced with 20-50 mL of whole blood in order to avoid excess hemodilution. Induction of hypo¬thermia during CPB reduces demand for O2 and preserves peripheral tissues. This is achieved by combination of body-surface cooling and use of a heat exchanger within the CPB circuit. Use of both techniques simultaneously maximizes control of the body temperature. When used in conjunction with anesthesia, hypothermia limits damage during CPB by maintaining low blood circulation.18,24
Cannulation for CPB
In preparation for CPB, the left carotid artery and the jugular vein are surgically isolated and the thoracic cavity is opened at the left 4th or 5th intercostal space. Heparin (400 U/kg) is adminis¬tered intravenously. Once the activated clotting time has exceeded 300 s, an arterial cannula is inserted into the carotid artery.25 In dogs weigh¬ing <5 kg, arterial cannulation to the carotid artery requires a 6-Fr cannula, whereas an 8-Fr cannula is needed for dogs weighing 5-10 kg. A single venous cannula is inserted into the left jugular vein. An 8 to 10-Fr cannula in dogs weighing <5 kg or a 10 to 14-Fr cannula in dogs weighing 5-10 kg is appropriate. Cannulation of peripheral vessels is preferred over direct can- nulation of aorta and right atrium in small dogs in order to avoid obstructing visualization of the surgical field by the cannulae. The CPB circuit is connected to both the arterial and venous cannulae.
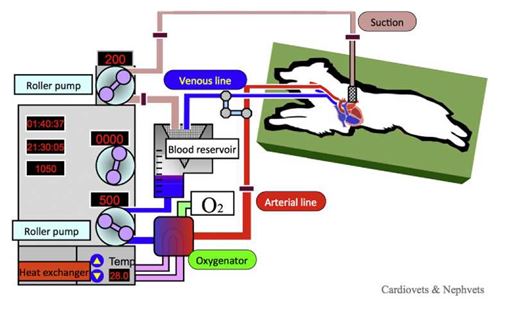
Air is carefully removed from the arterial side of the circuit. The artery and vein lines are de-clamped and CPB is commenced at a flow rate of 90-120 mL/kg/min. The body temperature is held between 25 and 30 °C. The pump flow is carefully monitored to match venous return (Fig. 1). During CPB, anesthesia is switched from inhalation of isoflurane to intravenous fen- tanyl and propofol infusion. During CPB, arterial and central venous blood pressure, oxygen saturation and blood gases are monitored.
Induction of cardiac arrest
Mitral valve repair requires a bloodless and motionless field of vision. This is achieved by infusing a cardioplegia solution into the coronary arteries via the aortic root. A catheter is inserted through a purse-string suture on the left lateral side of the ascending aorta. The aorta is occluded using a vascular clamp placed distal to the cardioplegia cannula and proximal to the brachioce¬phalic artery. Cardioplegic solution is immediately infused into root of the aorta to induce cardiac arrest (Video 1). Administration of cardioplegia solution is repeated every 20 min. Although this is the standard protocol for intracardiac procedures, it is also possible to utilize an on-beat cardiac surgery technique,17,21 which diminishes the risk of ischemia-reperfusion injury.26 However, this may cause blood to leak from the aorta obscuring the surgical field.
Figure 1 Cardiopulmonary bypass. An arterial cannula is placed in a carotid artery and a venous cannula is inserted into the jugular vein. Both cannulae were connected to the CPB circuit. Venous blood was withdrawn from the jugular cannula and sent from the blood reservoir to the artificial lung by the CPB pump; the blood was then returned to the carotid artery. Shed blood is returned to the blood reservoir via suction lines.
Discontinuing CPB
The patient is re-warmed to >36 °C prior to weaning from CPB. After the left atrium has been closed, the aortic clamp is removed. As coronary circulation restored, cardiac rhythm may return spontaneously (Video 3). Use of a defibrillator at 10—30 J may be required if ventricular fibrillation is observed. The flow rate is decreased in a stepwise fashion until CPB is terminated. Once the cannulae have been removed, it is generally necessary to administer protamine (1.0—1.5 mg of protamine per 100 units of heparin) to reverse the effects of heparin. Prot¬amine activates the complement cascade through the classic pathway and occasionally provokes temporary severe bronchospasm, elevation of pulmonary vascular resistance, and hypotension. These reactions can cause severe hypotension and hypoventilation. These adverse reactions are reduced by slow stepwise administration of prot¬amine (0.1—3.2 mg/kg per 5—20 min).
Mitral valve repair
Our team employs a left atriotomy approach to perform mitral valve repair . This enables visuali¬zation of ruptured mitral chordae tendineae through the opened left atrium (Video 2). Several methods of mitral repair have been reported in humans.3—6,27—29 The Carpentier technique, which uses leaflet resection and rigid or semi-rigid annuloplasty rings, has been widelyused in humans.27 However, this technique substantially disrupts important functions of the mitral valve complex, and as a result, most centers have found it difficult to repair more than 50—60% of insufficient valves.28,29 An alternative is the technique reported by Lawrie,28 who used artificial chords and a flexible annuloplasty ring sutured in place with a running technique without leaflet resection. This method can be correlated with the normally functioning mitral valve in the beating heart. Resection of the mitral leaflet would be a particularly difficult procedure in small-breed dogs because of their small size. These results suggest that mitral valve repair in dogs is best achieved with artificial chordal replacement and mitral annuloplasty without mitral resection.
Mitral chordal replacement
Replacement of chordae tendineae has been established as the most effective technique in repair of mitral chordal rupture; supplanting partial leaflet resection, chordal transfer, and chordal shortening.6,30-35 The main challenge in chordal replacement is the difficulty of adjusting artificial chordae to the appropriate length. Ideally artificial chordae length should match that of the opposing chordae to ensure proper coaptation of the opposing leaflets. Several methods, including application of a caliper, transesophageal echo¬cardiography, and multiple knots6,30,36,37 have been reported to ensure that artificial chordae are of the optimal length. However, these techniques may be difficult to apply to the hearts of small- breed dogs. Instead, a temporary Alfieri (edge-to- edge) stitch can be used to maintain the visual field and ensure proper coaptation of the leaf- lets.38 After observing a tendency toward over¬shortening of artificial chordae, we have success¬fully employed a temporary Alfieri stitch to place artificial chordae in the septal and mural mitral leaflets. Placement of artificial chordae is accom¬plished by passing a double-armed expanded-pol- ytetrafluoroethylene (ePTFE) suture (CV-6) through lateral portion of the septal leaflet and then though the craniolateral papillary muscle. The double-armed suture is then passed back through the septal leaflet. We now prefer to use a pledget in the papillary muscle as we have observed papillary muscle rupture in one patient. We then place a second ePTFE chordae tendineae between the medial portion of the septal leaflet and the caudomedial papillary muscle (Fig. 2, Video 2). A third ePTFE suture is placed between the mid-portion of the mural mitral leaflet and the caudomedial papillary. After all the artificial chordae are placed, a temporary Alfieri stitch is placed and the artificial chordae are adjusted for length and tied.
Mitral annuloplasty
Mitral annuloplasty plays an important role in maintaining long-term durability after mitral valve repair.39 The saddle-shaped mitral annulus is higher at the cranial and caudal segments and lower at the commissures.40-42 The mitral area is reduced from mid-diastole to late systole, such that the mitral annulus exhibits translational motion during systole.40,43 This sphincter mechanism increases the depth of leaflet coaptation during systole, and increases the annular orifice area during dia- stole.40-42 These physiological motions of the annulus should be maintained during mitral annu- loplasty. Annular stabilization with prosthetic materials enhances durability by increasing leaflet coaptation and preventing future annular dilatation.39
Figure 2 Placement of artificial chordae. The septal mitral leaflet is shown with ruptured caudal chordae tendineae and papillary muscles (A). The double-armed ePTFE suture is passed through the mitral valve, into the caudomedial papillary muscle, and then back through the mitral leaflet (B). Another suture was made on the other side in the same manner and both were tied. This procedure was repeated on the mural leaflet.

Saddle-shaped annuloplasty rings provide superior uniform annular force distribution compared to flat rings, and appear to minimize out- of-plane forces that could be transmitted to leaflets and chords.44 Prosthetic rings designed for humans are not of a suitable size for small-breed dogs. Use of a mitral plication suture instead of an annulo¬plasty ring may preserve the natural shape and hemodynamic performance of the annulus.45,46 However this method is not as durable as ring annuloplasty due to detachment of the suture from the annulus.47-50 By contrast, prosthetic soft ring annuloplasty provides reproducible and long-term results in dogs. The best alternative for small dogs is a soft prosthetic ring made of ePTFE material that can be trimmed to an appropriate size at surgery. The size of the mitral annulus can be determined by a sizer that matches the mitral annulus and the root of the aorta.
We frequently observe annular dilatation at the cranial and caudal mitral commissures. This prevents us from achieving proper coaptation, thereby leading to nontrivial mitral regurgitation. This may be rectified by use of a plication stitch between the mural and septal leaflets at the commissures.51 Our preferred approach for mitral annuloplasty is placement of plication sutures with pledgets at the cranial and caudal commissures of the mitral annulus to reduce the size of both commissure regions. Then 5-0 sutures are pre¬placed in the mitral annulus and passed through a strip of ePTFE material approximately 1.5 mm wide and 40-55 mm in length (Fig. 3, Video 3). The sutures are then tied to seat the annuloplasty ring. Antithrombotic therapy with dalteparin sodium should be started when the volume of drainage from the thoracic cavity is less than 3 mL/h and continued for 1 week. Administration of an antiplatelet agent is necessary for 1 -3 months after surgery.
Surgical outcomes
Mitral valve repair improves clinical signs, such as cough, dyspnea, and anorexia. Improved appetite can lead to gains in body weight and improved cardiac cachexia (Fig. 4). Auscultation should reveal significant reductions in the grade of the cardiac murmur. Chest X-rays should reveal significant decreases in vertebral heart size and tracheal elevation (Fig. 5). Echocardiography should show decreases in the left atrial and left ventricular end-diastolic diameters, with marked reductions in mitral regurgitation (Video 4). Mitral valve repair improves clinical signs and reverses cardiac remodeling. These outcomes reduce the need for cardiovascular drug therapy. Cumula¬tively, the improved clinical condition of surgically treated dogs indicates that mitral regurgitation can be effectively treated with mitral valve repair.
If left untreated, severe mitral regurgitation has a poor prognosis in canine patients.1 In most cases, dogs are not expected to survive for >1 year, even mitral valve repair.51 Other surgeons have re¬ported long-term survival (>3 years) after mitral valve repair.14
Figure 3 Mitral annuloplasty techniques.
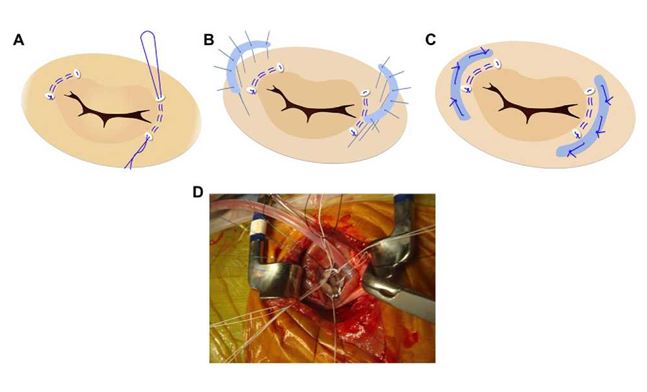
Mattress sutures with pledgets are placed in the cranial and caudal commissure of the mitral annulus to reduce the size of both commissure regions (A). Interrupted sutures are placed in the mitral annulus and passed through strips of ePTFE material (B). Annuloplasty is completed by tying the sutures and seating the annuloplasty rings (C). Intraoperative mitral annuloplasty is shown (D).
Figure 4 Dog with severe mitral regurgitation before and after mitral valve repair.
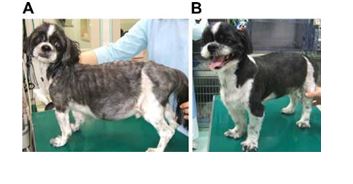
Prior to surgery, the dog was thin, had a poor hair coat and abdominal distension (A). Six months after surgery, the dog had gained weight, its coat condition had improved, and the abdomen distension had resolved (B).
if given medical treatment.1,2 Mitral valve repair and replacement, however, both improve prog¬nosis in dogs with mitral regurgitation.10,12,13,16,17 We observed 93% survival at 38 months after
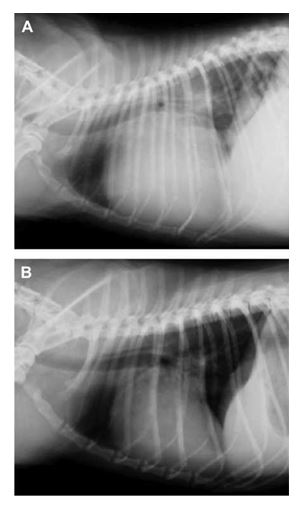
Figure 5 Thoracic radiographs before (A) and after (B) mitral valve repair demonstrating significant decrease in cardiac size.
Conclusion
Mitral valve repair in dogs using cardiopulmonary bypass is an effective treatment for severe mitral regurgitation in small dogs. Reestablishment of coaptation by annuloplasty and chordal replace¬ments using ePTFE are the most appropriate repair methods for small dogs. Successful mitral repair offers the probably of decreased clinical signs, reduced need for cardiac medical therapies, and improved survival in dogs with severe mitral regurgitation.
Conflict of interest
The author has no conflicts of interest to declare.
Acknowledgments
The author acknowledges surgical team members. This study was supported in part by a Grant-in-Aid for General Scientific Research (C-22580369) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; a grant from the Ministry of Health, Labour and Welfare, Japan; and a NUBS Research Grant in both 2009 and 2011.
References
- Serres F, Chetboul V, Tissier R, Sampedrano CC, Gouni V, Nicolle AP, Pouchelon JL. Chordae tendineae rupture in dogs with degenerative mitral valve disease: prevalence, survival, and prognostic factors (114 cases, 2001-2006). J Vet Intern Med 2007;21:258-264.
- Haggstrom J, Boswood A, O'Grady M, Jos O, Smith S, Swift S, Borgarelli M, Gavaghan B, Kresken JG, Patteson M, Ablad B, Bussadori CM, Glaus T, Kovacevi A, Rapp M, Santilli RA, Tidholm A, Eriksson A, Belanger MC, Deinert M, Little CJ, Kvart C, French A, R0n-Landbo M, Wess G, Eggertsdottir AV, O'Sullivan ML, Schneider M, Lombard CW, Dukes-McEwan J, Willis R, Louvet A, DiFruscia R. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med 2008;22:1124-1135.
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgi¬tation. Lancet 2009;373:1382-1394.
- Miura T, Eishi K, Yamachika S, Yamachika S, Hashizume K, Yamane K, Tanigushi S, Tanigawa K, Hashimoto W, Odate T, Nakaji S. Mitral valve repair for degenerative disease with Mitral valve repair in dogs leaflet prolapse: to improve long-term outcomes. Gen Thorac Cardiovasc Surg 2009;57:10-21.
- Schwartz CF, Grossi EA, Ribakove GH, Ursomanno P, Gogoladze G, Culliford AT, Galloway AC, Grossi EA. Ten-year results of folding plasty in mitral valve repair. Ann Thorac Surg 2010;89:485-488.
- Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med 2009;36:2261-2269.
- Klement P, Feindel CM, Scully HE, Mesher E, Klement G, Del Nido P, Wilson GJ. Mitral valve replacement in dogs. Surgical technique and postoperative management. Vet Surg 1987;16:231-237.
- Kanemoto I, Shibata S, Noguchi H, Chimura S, Kobayashi M, Shimizu Y. Successful mitral valvuloplasty for mitral regur¬gitation in a dog. Nihon Juigaku Zasshi 1990;52:411-414.
- Breznock EM. Tricuspid and mitral valvular disease: valve replacement. Semin Vet Med Surg (Small Anim) 1994;9: 234-239.
- White RN, Stepien RL, Hammond RA, Holden DJ, Torrington AM, Milner HR, Cobb MA, Hellens SH. Mitral valve replacement for the treatment of congenital mitral dysplasia in a bull terrier. J Small Anim Pract 1995;36: 407-410.
- Boggs LS, Dewan SJ, Ballard SE. Mitral valve reconstruction in a toy-breed dog. J Am Vet Med Assoc 1996;209:1872-1876.
- White RN, Boswood A, Garden OA, Hammond RA. Surgical management of subvalvular aortic stenosis and mitral dysplasia in a golden retriever. J Small Anim Pract 1997;38: 251-255.
- Buchanan JW, Sammarco CD. Circumferential suture of the mitral annulus for correction of mitral regurgitation in dogs. Vet Surg 1998;27:182-193.
- Griffiths LG, Orton EC, Boon JA. Evaluation of techniques and outcomes of mitral valve repair in dogs. J Am Vet Med Assoc 2004;224:1941-1945.
- Borenstein N, Daniel P, BehrL, Pouchelon JL, Carbognani D, Pierrel A, Macabet V, Lacheze A, Jamin G, Carlos C, Chetboul V, Laborde F. Successful surgical treatment of mitral valve stenosis in a dog. Vet Surg 2004;33:138-145.
- Orton EC, Hackett TB, Mama K, Boon JA. Technique and outcome of mitral valve replacement in dogs. J Am Vet Med Assoc 2005;226:1508-1511.
- Behr L, Chetboul V, Sampedrano CC, Vassiliki G, Pouchelon JL, Laborde F, Borenstein N. Beating heart mitral valve replacement with a bovine pericardial bioprosthesis for treatment of mitral valve dysplasia in a Bull Terrier. Vet Surg 2007;36:190-198.
- Kanemoto I, Taguchi D, Yokoyama S, Mizuno M, Suzuki H, Kanamoto T. Open heart surgery with deep hypothermia and cardiopulmonary bypass in small and toy dogs. Vet Surg 2010;39:674-679.
- Yamano S, Uechi M, Tanaka K, Hori Y, Ebisawa T, Harada K, Mizukoshi T. Surgical repair of a complete endocardial cushion defect in a dog. Vet Surg 2011;40:408-412.
- Uechi M, Mizukoshi T, Mizuno T, Mizuno M, Harada K, Ebi- sawa T, Takeuchi J, Sawada T, Uchida S, Shinoda A, Kasuya A, Endo M, Nishida M, Kono S, Fujiwara M, Nakamura T. Mitral valve repair under cardiopulmonary bypass in small breed dogs. J Am Vet Med Assoc, in press.
- Uechi M, Harada K, Mizukoshi T, Mizuno T, Mizuno M, Ebisawa T, Ohta Y. Surgical closure of an atrial septal defect using cardiopulmonary bypass in a cat. Vet Surg 2011;40: 413-417.
- Takashima K, Soda A, Tanaka R, Yamane Y. Long-term clinical evaluation of mitral valve replacement with porcine bioprosthetic valves in dogs. J Vet Med Sci 2008;70: 279-283.
- Doenst T, Borger MA, David TE. Long-term results of bio¬prosthetic mitral valve replacement: the pericardial perspective. J Cardiovasc Surg (Torino) 2004;45:449-454.
- Lew LJ, Fowler JD, Egger CM, Thomson DJ, Rosin MW, Pharr JW. Deep hypothermic low flow cardiopulmonary bypass in small dogs. Vet Surg 1997;26:281-289.
- Griffiths LG. Surgery for cardiac disease in small animals: current techniques. Vet Clin North Am Small Anim Pract 2010;40:605-622.
- Matsumoto Y, Watanabe G, Endo M, Sasaki H, Kasashima F, Kosugi I. Efficacy and safety of on-pump beating heart surgery for valvular disease. Ann Thorac Surg 2002;74: 678-683.
- Carpentier A, Relland J, Deloche A, Fabiani JN, D'Allaines C, Blondeau P, Piwnica A, Chauvaud S, Dubost C. Conservative management of the prolapsed mitral valve. Ann Thorac Surg 1978;26:294-302.
- Lawrie GM. Structure, function, and dynamics of the mitral annulus: importance in mitral valve repair for myxomatous mitral valve disease. Methodist Debakey Cardiovasc J 2010; 6:8-14.
- Ben Zekry S, Lang RM, Sugeng L, McCulloch ML, Weinert L, Raman J, Little SH, Xu J, Lawrie GM, Zoghbi WA. Mitral annulus dynamics early after valve repair: preliminary observations of the effect of resectional versus non¬resectional approaches. J Am Soc Echocardiogr 2011;24: 1233-1242.
- Bizzarri F, Tudisco A, Ricci M, Rose D, Frati G. Different ways to repair the mitral valve with artificial chordae: a systematic review. J Cardiothorac Surg 2010;5:22.
- Falk V, Seeburger J, Czesla M, Borger MA, Willige J, Kuntze T, Doll N, Borger F, Perrier P, Mohr FW. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Car- diovasc Surg 2008;136:1205. discussion 1205-1206.
- Gammie JS, Sheng S, Griffith BP, Peterson ED, Rankin JS, O'Brien SM, Brown JM. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009;87: 1431-1437.
- Adams DH, Kadner A, Chen RH. Artificial mitral valve chordae replacement made simple. Ann Thorac Surg 2001; 71:1377-1378. discussion 1378-1379.
- Kasegawa H, Shimokawa T, Shibazaki I, Hayashi H, Koyanagi T, Ida T. Mitral valve repair for anterior leaflet prolapse with expanded polytetrafluoroethylene sutures. Ann Thorac Surg 2006;81:1625-1631.
- Kudo M, Yozu R, Kokaji K, Iwanaga S. Feasibility of mitral valve repair using the loop technique. Ann Thorac Car- diovasc Surg 2007;13:21-26.
- Calafiore AM. Choice of artificial chordae length according to echocardiographic criteria. Ann Thorac Surg 2006;81: 375-377.
- Ruyra-Baliarda X. Preliminary experience with the no prolapse system. A new device for ensuring the proper length of artificial chordae in mitral valve repair. Interact Cardiovasc Thorac Surg 2010;10:165-167.
- Morimoto H, Tsuchiya K, Nakajima M, Akashi O, Kato K. Mitral valve repair for extended commissural prolapse involving complex prolapse. Asian Cardiovasc Thorac Ann 2007;15:210-213.
- Gillinov AM, Tantiwongkosri K, Blackstone EH, Houghtaling PL, Nowicki ER, Sabik 3rd JF, Johnston DR, Svensson LG, Mihaljevic T. Is prosthetic annuloplasty necessary for durable mitral valve repair? Ann Thorac Surg 2009;88:76-82.
- Komeda M, Glasson JR, Bolger AF, Daughters 2nd GT, Niczyporuk MA, Ingels Jr NB, Miller DC. Three-dimensional dynamic geometry of the normal canine mitral annulus and papillary muscles. Circulation 1996;94:II159—163.
- Hoole SP, Liew TV, Boyd J, Wells FC, Rusk RA. Transthoracic real-time three-dimensional echocardiography offers addi¬tional value in the assessment of mitral valve morphology and area following mitral valve repair. Eur J Echocardiogr 2008;9:625—630.
- Nguyen TC, Itoh A, Carlhall CJ, Bothe W, Timek TA, Ennis DB, Oakes RA, Liang D, Daughters GT, Ingels Jr NB, Miller DC. The effect of pure mitral regurgitation on mitral annular geometry and three-dimensional saddle shape. J Thorac Cardiovasc Surg 2008;136:557—565.
- Tsakiris AG, Von Bernuth G, Rastelli GC, Rastelli GC, Bourgeois MJ, Titus JL, Wood EH. Size and motion of the mitral valve annulus in anesthetized intact dogs. J Appl Physiol 1971;3:611—618.
- Jensen MO, Jensen H, Smerup M, Levine RA, Yoganathan AP, Nygaard H, Hasenkam JM, Nielsen SL. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation 2008; 118:S250—S255.
- Borghetti V, Campana M, Scotti C, Domenighini D, Totaro P, Coletti G, Pagani M, Lorusso R. Biological versus prosthetic ring in mitral-valve repair: enhancement of mitral annulus dynamics and left-ventricular function with pericardial annuloplasty at long term. Eur J Cardiothorac Surg 2000;17: 431—439.
- Detter C, Aybek T, Kupilik N, Fischlein T, Moritz A. Mitral valve annuloplasty: comparison of the mural annulus shortening suture (MASS) with the Carpentier-Edwards prosthetic ring. J Heart Valve Dis 2000;9:478—486.
- Duebener LF, Wendler O, Nikoloudakis N, Georg T, Fries R, Schaers HJ. Mitral-valve repair without annuloplasty rings: results after repair of anterior leaflet versus posterior- leaflet defects using polytetrafluoroethylene sutures for chordal replacement. Eur J Cardiothorac Surg 2000;17: 206—212.
- Aybek T, Risteski P, Miskovic A, Simon A, Dogan S, Abdel- Rahman U, Moritz A. Seven years' experience with suture annuloplasty for mitral valve repair. J Thorac Cardiovasc Surg 2006;131:99—106.
- Nagy ZL, BodiA, VaszilyM, SzerafinT, Horvath A, PeterffyA. Five-year experience with a suture annuloplasty for mitral valve repair. Scand Cardiovasc J 2000;34:528—532.
- Komoda T, Hubler M, Siniawski H, Hetzer R. Annular stabi¬lization in mitral repair without a prosthetic ring. J Heart Valve Dis 2000;9:776—782.
- Burr LH, Krayenbuhl C, Sutton MS. The mitral plication suture: a new technique of mitral valve repair. J Thorac Cardiovasc Surg 1977;73:589—595.
^Наверх









