Left subclavian artery dissection associated with connective tissue abnormalities resembling Marfan-like syndrome in an English bulldog

Author information
Biasato I., Zanatta R., Maniscalco L., Evangelista R., Iotti B., Iussich S. Left subclavian artery dissection associated with connective tissue abnormalities resembling Marfan-like syndrome in an English bulldog // J Vet Cardiol. 2018 Apr;20(2):136-142.
Abstract
The unexpected demise of a 12-year-old male neutered English bulldog solicited a gross examination, which revealed a blood-filled space occurring in the proximal left subclavian artery (LSA). It originated about 1 cm from the branching point of the vessel and progressively dilated for 3 cm distal to this origin. Histopathological investigation showed that the tunica media of the LSA was more than 50% split, with the blood-filled space dissecting through the arterial wall. In the tunica media of the LSA, severe multifocal fragmentation and/or loss of the elastic fibers was observed. The retained disorganized elastic fibers were separated and disoriented due to accumulations of acid mucopolysaccharide. Marked, diffuse medial, and adventitial fibrous tissue deposition was also identified. The cause of death was attributed to acute hemorrhagic and necrotizing pancreatitis with pulmonary edema, suggesting that LSA dissection was an incidental finding. Subclavian artery dissection is extremely rare in humans, where the involvement of the LSA in cases of aortic dissection both with or without Marfan syndrome has been reported. Aortic and pulmonary artery dissection in bovines and aortic aneurysm and dissection in dogs have been reported to be associated with Marfan and Marfan-like syndromes, respectively. Histopathological findings suggestive of underlying connective tissue abnormalities resembling Marfan-like syndrome (i.e., the appearance of the elastic tissue and the degenerative changes of the tunica media) were detected in the first case of LSA dissection in dogs and veterinary medicine, herein described.
KEYWORDS: Canine; Elastic tissue; Heart; Medial degeneration; Mucopolysaccharide
A 12-year-old male neutered English bulldog weighing 19.8 kg was referred for a routine follow¬up visit at the Veterinary Teaching Hospital of the Department of Veterinary Sciences (University of Turin). A diagnosis of leishmaniasis had been made nearly 2-and-a-half years before, and the animal was clinically cured using miltefosine (2 mg/kg PO q 24 h, for 28 days) and allopurinol (20 mg/kg PO q 24 h, throughout the follow-up period). During the follow-up period, the dog did not show any clinical symptoms indicative of a renewed clinically active leishmaniasis. During the last visit, the owners reported occasional episodes of nausea and vomiting, but the animal appeared to be in overall good health conditions. General physical exami¬nation and blood analyses (complete blood count, biochemical analysis, and serum protein electrophoresis) showed no significant abnormalities. In the days after the visit, the owners observed a spontaneous resolution of the symptoms and the dog stayed healthy for the following two months. Unexpectedly, the animal was found dead by the owners at the end of the second month. A written consent for necropsy was signed by the owners and a complete post mortem investigation was performed.
At post mortem examination, the left sub¬clavian artery (LSA) was moderately and diffusely dilated, starting about 1 cm distal to its branching point (Fig. 1A). Multifocal to coalescing ecchymotic hemorrhages were also observed on the adventitial surface of the vessel wall (Fig. 1B). Serial cross sections of the dilated proximal seg¬ment of the LSA revealed a longitudinal blood-filled space affecting more than 50% of the arterial wall. This blood-filled space progressively dilated lengthwise for 3 cm distal to its origin (Fig. 1C—D). The aorta and pulmonary artery showed no significant gross abnormalities. To evaluate all cardiac structures, the heart was externally examined and opened following the inflow and outflow tracts after transverse sectioning at the level of the middle third of the ventricles. Heart weight (163.3 g) and its percentage relative to body weight (0.82%) fell within the normal reference values reported in adult dogs [1], presenting no signs of cardiac hypertrophy.
Both atria and ventricles were not dilated and the atrio-ventricular and semilunar valves showed normal morphology. The lungs were severely and diffusely enlarged, dark red, wet, and exuded fluid on sectioning. The pancreatic parenchyma was markedly and diffusely red with multifocal to coalescing ecchymotic hemorrhages being observed throughout the cut surfaces. The surrounding peripancreatic mesenteric adipose tissue displayed fat necrosis with saponification. Both kidneys were moderately and diffusely pale and firmer than normal. No significant gross abnormalities were detected in other organs. Samples of LSA, aorta, pulmonary artery, heart, lungs, spleen, liver, kidney, stomach, intestine, thyroids, adrenal glands, and pancreas were collected and fixed in a 10% buffered formalin solution. Organ samples were successively processed by routine methods, embedded in paraffin wax blocks, sectioned at 5 pm thickness and stained with Haematoxylin & Eosin. Selected serial sections of the LSA were also stained with Masson’s Trichrome, Elastic Red Picro Sirius, and Alcian Blue/Periodic Acid-Schiff to investigate collagen, elastic fibers, and mucopo¬lysaccharide deposition. Tissue sections were examined by light microscopy. The tunica media of the LSA was split for more than 50% of its thickness, with the blood-filled space dissecting through the arterial wall (Fig. 2A).
In the tunica media of both dissected and non-dissected regions of the LSA, severe multifocal fragmentation and/ or loss of the elastic fibers was observed (Fig. 2B). The retained disorganized elastic fibers were separated and further disoriented due to accumulations of acid mucopolysaccharide (Fig. 2C). Within the tunica media of the dissected regions of LSA, marked and diffuse fibrous tissue deposition was identified, along with smooth muscle nuclei loss and moderate multifocal chondroid metaplasia. The tunica adventitia of the dissected LSA also showed severe diffuse deposition of tight and concentric fibrous tissue (Fig. 2D). In the tunica intima of the dissected and non-dissected LSA, moderate diffuse fibromuscular hyperplasia was observed. No significant alterations were detected in the aorta and pulmonary artery. The lungs showed marked diffuse bronchoalveolar edema associated with mild-to-severe, diffuse con¬gestion. Within the pancreas, severe multifocal interstitial and lobular hemorrhagic necrosis with neutrophilic inflammation was detected. Marked multifocal necrosis of the peripancreatic mesenteric adipose tissue was also observed. Finally, kidneys showed moderate multifocal segmental proliferative glomerulonephritis (affecting 20% of glomeruli). Periodic Acid-Schiff staining was performed to exclude any thickening of the glomerular basal membrane and to confirm the proliferative changes detected on Haematoxylin & Eosin. Moderate multifocal interstitial lymphoplasmacytic inflammation was also observed in the kidneys, associated with interstitial fibrous tissue deposition and tubular dilation.
Figure 1 Gross examination of the heart. A) Dorsolateral view of the heart base. The left subclavian artery (LSA) is moderately and diffusely dilated
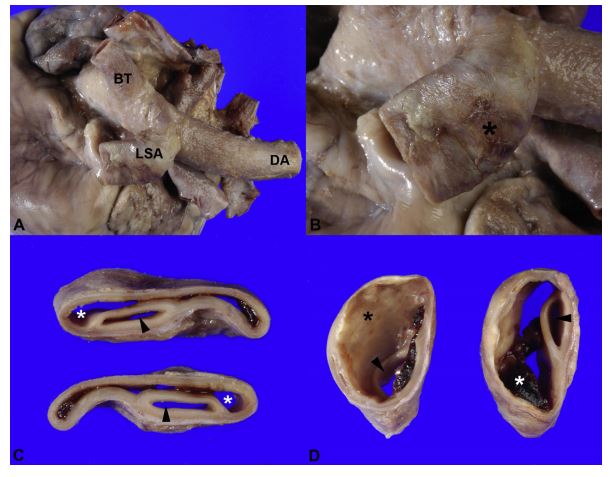
B) Closer view of the left subclavian artery. Large and multifocal hemorrhages (*) on the adventitial surface of the wall are observed. C) Serial cross sections of the proximal segment of left subclavian artery. The wall of the artery is split for more than 50% of its thickness by blood-filled space (*) dissecting through the arterial wall. Arrowheads = true lumen of the vessel. D) The dissection (*) progressively dilates distal to its origin. Arrowheads = true lumen of the vessel. BT, brachiocephalic trunk; DA, descending aorta.
No significant alterations were detected in the other organs. For reference, a control sample, such as the 6-year-old dog presented in Fig. 3A—B, shows elastic fibers in the tunica media arranged in regular thin longitudinal bundles, with no mucopolysaccharide deposits.
Based on gross and histopathological findings, a diagnosis was made of LSA dissection with concurrent acute hemorrhagic and necrotizing pancreatitis and pulmonary edema. The cause of death was attributed to the pancreatic and pulmonary abnormalities.
Discussion
Dissection of the great arteries (i.e., aorta and pulmonary artery) is an uncommon finding in ani¬mals. Aortic and pulmonary artery dissection have been recognized as clinicopathological entities in dogs [2—7], cats [8,9], and cattle [10]. Aortic dis¬section in particular is generally attributed to systemic hypertension [2,4,8], aortitis (sometimes associated with Spirocerca lupi), degenerative processes [3], or inheritable defects in the con¬nective tissue components, including elastin [3,10] or collagen fibrils [11]. Chetboul et al. [11] pre¬viously described familial aortic aneurysm with involvement of the LSA in leonberger dogs, but to the best of the authors’ knowledge, no reports of LSA dissection alone are currently available in veterinary medicine.
Subclavian artery dissection is rarely reported in humans and is usually associated with anomalies of the aortic arch [12], trauma [13], and iatrogenic injury during catheterization [14] or angioplasty [15]. Involvement of the LSA in cases of aortic dissection with or without Marfan syndrome has been also reported [16,17].
Figure 2 Histopathological examination of the left subclavian artery
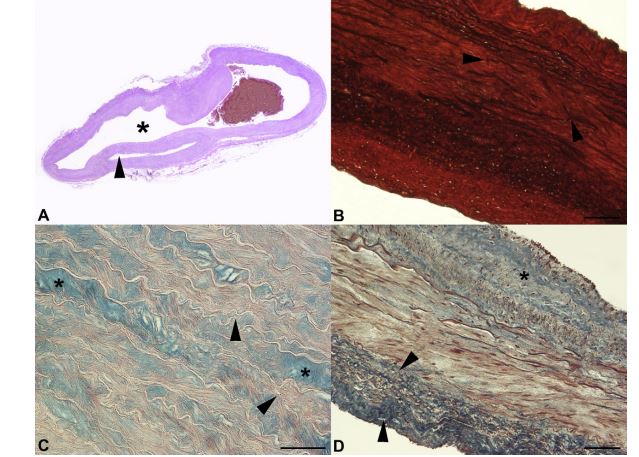
A) The tunica media of the left subclavian artery is split for more than 50% of its thickness, with a blood-filled space (*) dissecting through the arterial wall. Arrowhead = true lumen of the vessel (Haematoxylin & Eosin stain, photograph of the glass slide). B) The tunica media of the vessel shows marked multifocal fragmentation and/or loss of the elastic fibers (arrowheads; Elastic Red Picro Sirius stain, 10x magnification, bar = 100 mm). C) In the tunica media of the artery, severe multifocal acid mucopolysaccharide accumulation (*) between the disorganized elastic fibers (arrowheads) is observed (Alcian Blue/ Periodic Acid-Schiff stain, 20x magnification, bar = 100 mm). D) The tunica adventitia of the artery shows marked diffuse fibrous tissue deposition (arrowheads). Medial fibrosis (*) is evident (Masson’s Trichrome stain, 10x magnification, bar = 100 mm). Masson’s Trichrome = collagen and muscle stain blue and red, respectively. Elastic Red Picro Sirius stain = collagen and elastic fibers stain red and black, respectively. Alcian Blue/Periodic Acid-Schiff stain = neutral and acid mucopolysaccharide stain purple and blue, respectively.
Figure 3 Histopathological examination of the left subclavian artery from an adult control dog
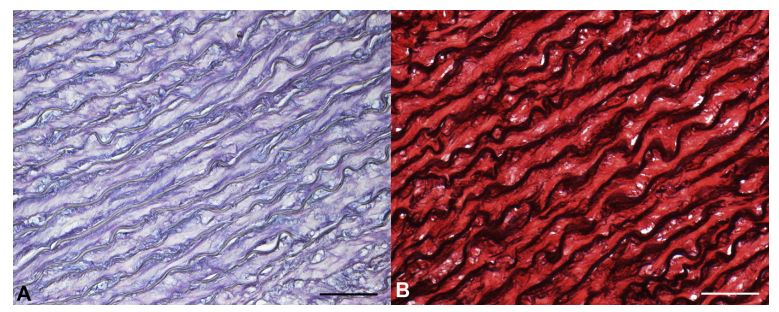
A) In the tunica media, no mucopolysaccharide matrix is observed (Alcian Blue/Periodic Acid-Schiff stain, 20x magnification, bar = 100 mm). B) The tunica media shows regular longitudinal arrangement of the elastic fibers (Elastic Red Picro Sirius stain, 20x magnification, bar = 100 mm). Elastic Red Picro Sirius stain = collagen and elastic fibers stain red and black, respectively. Alcian Blue/Periodic Acid-Schiff stain = neutral and acid mucopolysaccharide stain purple and blue, respectively.
The canine histopathological findings described in the present report, especially the appearance of the elastic tissue (disorganization, fragmentation, and/or loss of the elastic fibers) and the degenerative changes of the tunica media (mucopolysaccharide accumulations), are suggestive of underlying connective tissue abnormalities resembling the Marfan-like syndrome previously reported in canine aortic aneurysm [11] and dissection [3,7]. Marfan syndrome is only recognized in humans [18] and bovines [10].
In the former, it is a connective tissue disorder inherited in an autosomal dominant manner and caused mainly by a heterozygous mutation in the gene that encodes fibrillin-1, a glycoprotein involved in the con¬struction of microfibrils necessary to form elastic fibers [18]. Gross and microscopic cardiovascular lesions described in bovine Marfan syndrome are largely similar, but cystic medial necrosis/degeneration, as described in humans, is not reported in affected bovine aortae [10].
Despite the similarities between the histopathological findings, a confident diagnosis of Marfan syndrome in veterinary medicine seems difficult due to the ill-defined characteristics of the disease and the lack of available genetic testing [7]. Another interesting aspect to consider is that several human diseases, genetic syndromes (Marfan syndrome included), and vasculopathic aging processes involved in vascular injury can cause both distinct and nonspecific histopathologic changes, with degeneration of the tunica media as a common characteristic.
Consequently, no specific terms have gained full acceptance, and inaccurate terms (i.e., 'cystic medial necrosis/degeneration’) are often used to indicate several histopathological findings, thus leading to confusion and inconsistencies in diagnosis [19]. The heterogeneity in defining this pathology can be also identified in Marfan-like syndrome in dogs. Chetboul et al. [11] and Boulineau et al. [3] used both 'cystic medial degeneration’ and 'cystic medial necrosis’ to describe the presence of disorganized and fragmented elastic fibers surrounding cystic or pseudocystic spaces that contain acid mucopolysaccharide or amorphous eosinophilic material. On the contrary, Lenz et al. [7] preferred the term 'mucinous degeneration’ to describe the basophilic mucopolysaccharide matrix collected between disorganized elastin fibers. A recent consensus document has proposed a revised, unified nomenclature for the variety of noninflammatory degenerative aortic pathologies in humans, replacing older terms such as 'cystic medial necrosis/degeneration’ by more technically accurate terms such as 'mucoid extracellular matrix accumulation’, 'elastic fiber fragmentation and/or loss’, and 'smooth muscle cell nuclei loss’ [19]. Considering the relevant similarities between the histopathological findings of Marfan and Marfan-like syndromes in humans and animals, the importance of a standard, more precise terminology could be investigated in veterinary medicine.
As opposed to human patients with Marfan syndrome, in which the involvement of LSA in cases of aortic dissection is frequent [16,17], the dog of the present report developed LSA dissection with no involvement of the aorta. In people with spontaneous subclavian artery dissections in the absence of aortic involvement, hypertension-induced stress has been hypothesized as a potential cause [20]. However, no past medical history of systemic hypertension was found in the dog described in the present report. An association between magnitude of systemic hypertension and severity of adverse renal changes has previously been reported in dogs with induced renal failure [21].
In the present report, the identification of moderate (affecting less than 30% of renal tubules and glomeruli) renal post mortem alterations (which were ascribed to the previous leishmaniasis) was not considered severe enough to increase systemic arterial pressure. Furthermore, no histopathological changes typical of hypertensive angiopathy were observed in the arterial vessels. Another advanced hypothesis is that the incidence of dissection is related to the direct arterial pulsatile flow, with the LSA being thought to receive a stronger pulsatile flow than other arterial vessels [20]. In the dog of the present report, underlying connective tissue abnormalities and local hemodynamics may have caused focal weakening of the arterial wall, leading to mechanical stress and ultimately resulting in the dissection.
Medial fibrous tissue deposition, which was identified in the present report, is not a typical finding of Marfan and Marfan-like syndrome. The older age of the animal and the parallel detection of fibromuscular intimal hyperplasia may suggest underlying arteriosclerosis, which can alone be a cause of arterial dissection. However, the intimal changes were mild, and the medial fibrosis was observed only in the dissected segment of the LSA. The fibrous tissue deposition was also almost exclusively localized along the dissection plane, thus suggesting a chronicity of the dissection with a subsequent chronic repair process. Furthermore, the other arterial vessels showed no significant histopathological alterations. Since fibrosis can be a non-specific, end-stage change resulting from vessel injury, the hypothesis of chronic vasculitis (i.e., S. lupi or Leishmania infection) or previous unknown trauma should also be taken into account. However, the histopathological findings taken together were suggestive of an underlying connective tissue disorder.
The detection of fibrous tissue deposition in the tunica adventitia, which represents another unusual finding in Marfan and Marfan-like syndrome, was probably the consequence of the LSA dissection. Indeed, the tunica adventitia has been reported to respond to arterial wall injury by participating in arterial wall remodeling. In particular, adventitial fibrosis characterizes the response to mechanical stress (i.e., hypertension) and is involved in the constrictive remodeling of arterial segments [22].
Apart from Marfan and Marfan-like syndromes, disruption of connective tissue of large blood vessels in animals has also been reported in copper deficiency [23] and Ehlers-Danlos syndrome [24]. One of the hallmark gross features of severe cop¬per deficiency in animals is cardiac enlargement, often associated with pale color, textural softness, and ventricular aneurysms. Microscopically, fat and glycogen deposits and focal sites of myocardial inflammation, hemorrhages, necrosis, and fibrosis are frequently observed [23]. However, no such alterations were detected in the present case. Fragmentation and isolation of collagen fibers in the tunica media of the LSA by large clear and optically empty spaces with extravasated erythrocytes dissecting through them has recently been reported to cause vessel rupture in a dog with Ehlers-Danlos syndrome [24]. However, in the present report, the abnormalities were found in the structure and arrangement of elastic fibers, rather than of collagen fibrils.
No visible signs of vessel rupture or hemothorax were observed in our case, thus suggesting that the LSA dissection was not the cause of death. This is partially in agreement with what is observed in human patients, where sudden death due to an acute rupture of the subclavian artery is extremely rare [25].
To the best of authors’ knowledge, this is the first report of LSA dissection in dogs and in veterinary medicine. The associated histopathological findings are suggestive of an underlying connective tissue abnormalities resembling Marfan-like syndrome.
Conflicts of Interest Statement
The authors do not have any conflicts of interest to disclose.
References
- Northup DW, Van Liere EJ, Stickney JC. The effect of age, sex, and body size on the heart weight-body weight ratio in the dog. Anat Rec 1957;128:411-7.
- Waldrop JE, Stoneham AE, Tidwell AS, Jakowski RM, Rozanski EA, Rush JE. Aortic dissection associated with aortic aneurysms and posterior paresis in a dog. J Vet Intern Med 2003;17:223-9.
- Boulineau TM, Andrews-Jones L, Van Alstine W. Sponta¬neous aortic dissecting hematoma in two dogs. J Vet Diagn Invest 2005;17:492-7.
- Cohen JA, Bulmer BJ, Patton KM, Sisson DD. Aortic dis¬section associated with an obstructive aortic chon¬drosarcoma in a dog. J Vet Cardiol 2010;12:203-10.
- Markovic LE, Kellihan HB, Roldan-Alzate A, Drees R, Bjorling DE, Francois CJ. Advanced multimodality imaging of an anomalous vessel between the ascending aorta and main pulmonary artery in a dog. J Vet Cardiol 2014;16: 59-65.
- Scansen BA, Simpson EM, Lopez-Alvarez J, Thomas WP, Bright JM, Eason BD, Rush JE, Dukes-McEwan J, Green HW, Cunningham SM, Visser LC, Kent AM, Schober KE. Pulmo¬nary artery dissection in eight dogs with patent ductus arteriosus. J Vet Cardiol 2015;17:107-19.
- Lenz JA, Bach JF, Bell CM, Stepien RL. Aortic tear and dissection related to connective tissues abnormalities resembling Marfan syndrome in a Great Dane. J Vet Cardiol 2015;17:134-41.
- Wey AC, Atkins CE. Aortic dissection and congestive heart failure associated with systemic hypertension in a cat. J Vet Intern Med 2000;14:208-13.
- Biasato I, Tursi M, Zanet S, Longato E, Capucchio MT. Pulmonary artery dissection causing haemothorax in a cat: potential role of Dirofilaria immitis infection and literature review. J Vet Cardiol 2016;19:82-7.
- Potter KA, Besser TE. Cardiovascular lesions in bovine Marfan syndrome. Vet Pathol 1994;31:501-9.
- Chetboul V, Tessier D, Borenstein N, Delisle F, Zilberstein L, Payen G, Leglaive E, Franc B, Derumeaux G, Pouchelon JL. Familial aortic aneurysm in Leonberg dogs. J Am Vet Med Assoc 2003;223:1159-62.
- Guhathakurta S, Agarwal R, Borker S, Sharma AK. Chronic dissection of the left subclavian artery with pseudocoarctation. Tex Heart Inst J 2003;30:221-4.
- Scheffler P, Uder M, Gross J, Pindur G. Dissection of the proximal subclavian artery with consecutive thrombosis and embolic occlusion of the hand arteries after playing golf. Am J Sports Med 2003;31:137-40.
- Frohwein S, Ververis JJ, Marshall JJ. Subclavian artery dissection during diagnostic cardiac catheterization: the role of conservative management. Cathet Cardiovasc Diagn 1995;34:313-7.
- Schmitter SP, Marx M, Bernstein R, Wack J, Semba CP, Dake MD. Angioplasty-induced subclavian artery dissection in a patient with internal mammary artery graft: treat¬ment with endovascular stent and stent-graft. AJR Am J Roentgenol 1995;165:449-51.
- Yoshii S, Kamiya K, Matsukawa T, Ueno A. 3D analysis of the aortic arch aneurysm: importance of the transverse arch curve from the horizontal view. Nippon Geka Gakkai Zasshi 1988;89:972.
- Valentine RJ, Boll JM, Hocking KM, Curci JA, Garrard CL, Brophy CM, Naslund TC. Aortic arch involvement worsens the prognosis of type B aortic dissections. J Vasc Surg 2016; 64:1212-8.
- Canadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. Part 1: pathophysiology and diagnosis. Nat Rev Cardiol 2010;7:256-65.
- Halushka MK, Angelini A, Bartoloni G, Basso C, Batoroeva L, Bruneval P, Buja LM, Butany J, d'Amati G, Fallon JT, Gallagher PJ, Gittenberger-de Groot AC, Gouveia RH, Kholova I, Kelly KL, Leone O, Litovsky SH, Maleszewski JJ, Miller DV, Mitchell RN, Preston SD, Pucci A, Radio SJ, Rodriguez ER, Sheppard MN, Stone JR, Suvarna SK, Tan CD, Thiene G, Veinot JP, van der Wal AC. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovasc Pathol 2016;25:247-57.
- Iwamuro Y, Nakahara I, Tanaka M, Higashi T, Watanabe Y, Harada K, Fujimoto M, Oku T. Occlusion of the vertebral artery secondary to dissection of the subclavian artery-case report. Neurol Med Chir (Tokyo) 2005;45: 97-9.
- Finco DR. Association of systemic hypertension with renal injury in dogs with induced renal failure. J Vet Intern Med 2004;18:289-94.
- Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol 2007;27:1259-68.
- Saari JT, Schuschke DA. Cardiovascular effects of dietary copper deficiency. Biofactors 1999;10:359-75.
- Uri M, Verin R, Ressel L, Buckley L, McEwan N. Ehlers- Danlos syndrome associated with fatal spontaneous vas¬cular rupture in a dog. J Comp Path 2015;152:211-6.
- Garewal M, Selhorst JB. Subclavian artery dissection and triple infarction of the nervous system. Arch Neurol 2005; 62:1917-9.
^Наверх









