Cardiac troponin I concentrations in normal dogs and cats using a bedside analyzer

Author information
Adin D.B., Milner R.J., Berger K.D., Engel C., Salute M. Cardiac troponin I concentrations in normal dogs and cats using a bedside analyzer // J Vet Cardiol. 2005 May;7(1):27-32.
Abstract
OBJECTIVE: To develop reference ranges for cardiac troponin I (cTnI) in normal dogs and cats using the Biosite Triage Meter ((R)). BACKGROUND: Reference ranges for cTnI in dogs and cats have not yet been reported for this inexpensive bedside analyzer. METHODS: Purified free canine cTnI was diluted to 5 known concentrations to assess linearity and recovery. Interassay and intraassay precision were evaluated using 3 dilutions obtained from a dog with an elevated cTnI concentration. EDTA plasma was obtained from 55 normal dogs and 58 normal cats for analysis of cTnI. RESULTS: Measured values of purified cTnI closely matched calculated concentrations (r(2)=0.997) and recovery ranged from 107-164%. Intraassay precision was 2.76+/-1.20% and interassay precision was 8.50+/-4.19%. The dogs were 4.8+/-3.1 years and 24.4+/-11.2kg (27 Mc, 19 Fs, 5 M, 4 F). The cats were 4.9+/-2.8 years and 5.1+/-1.19kg (36 Mc, 22 Fs). The median and range (5th and 95th percentile) of cTnI for dogs were <0.05ng/mL (<0.05-0.12). The median cTnI for cats was <0.05ng/mL, as was the range, because only 3 cats (the upper 5% of the population) had detectable cTnI concentrations. The lower limit of detection for this assay is 0.05ng/mL. CONCLUSIONS: This study provides reference ranges for cTnI in dogs and cats using the Triage Meter((R)), an affordable bedside analyzer. The availability of reference ranges for this machine may increase clinical use and research of this marker in veterinary medicine.
Introduction
Troponins are myofibrillar proteins that regulate the calcium-mediated interaction between actin and myosin in both cardiac and skeletal muscle. The troponin complex consists of troponin I which inhibits actin and myosin interaction, troponin C which binds calcium to relieve inhibition by troponin I, and troponin T which binds to tropomyosin.1 Cardiac troponin I (cTnI) is the only one that is uniquely expressed in the myocardium.2 Both cardiac troponin I (cTnI) and cardiac troponin T have been widely recognized as highly sensitive and specific blood markers for the noninvasive diagnosis of increased cardio- myocyte permeability, and blood concentrations appear to correlate with the extent of myocardial injury in people and animals.3-11
Free intramyocardial cardiac troponin I (cTnI) within the cytosolic pool only accounts for about 2-4% of the total cardiomyocyte troponin I in dogs and can be released without histological evidence of myocardial cell injury, accounting for a low background level.3,6,7 Since the majority of cTnI is structurally bound within the cardiomyocyte, it is released into the circulation only after major injury with cell disruption and necrosis.6,7,12 Troponins are detectable in the blood 5-7 h after cardiac injury, peak at 1-2 days and dissipate by 1-2 weeks in human beings with acute myocardial infarction and in dogs with experimental myocardial infarction.1,13 The primary advantage of using cardiac troponins over more traditional markers of myocardial injury, such as CK-MB, is that they are more cardiospecific and the circulating concentration of cardiac troponin remains detectable for a longer period of time.1,12,13
Because of their high sensitivity and specificity, circulating cardiac troponins have become very important for the early detection of myocardial infarction in human beings.1,14 Although myocardial infarction is uncommon in veterinary medicine, some dogs and cats with conditions involving myocardial damage, such as hypertrophic cardiomyopathy, unclassified cardiomyopathy, dilated cardiomyopathy, subaortic stenosis, mitral valve degeneration, gastric dilatation-volvulus, blunt thoracic trauma and babesiosis have been found to have elevated cardiac troponin I (cTnI) concentrations .3-6,15,16
The molecular structure of troponin proteins is highly conserved across species, and some current assays developed for their detection in humans have been used and validated in several other species.2,17 Nucleotide sequencing of canine and feline cTnI has recently been published, demonstrating that canine cardiac troponin I (cTnI) showed 94% amino acid homology with human cTnI, feline cTnI showed 92% amino acid homology with human cTnI and canine cTnI showed 96% amino acid homology with feline cTnI.18 Lack of standardization among different assays for cTnI is problematic for its clinical use because there are numerous cTnI assays in the market, and up to a 10-fold difference in values can occur largely due to differences in antibodies used in assay calibration.19-21 Reference ranges have been established for dogs and cats using some machines, but because assays have not been standardized, they must be generated for each machine. The previously published papers establishing reference ranges in dogs and cats have used relatively expensive machines (all greater than 35,000 US dollars); the OPUS Immunoassay System,3 Stratus CS stat flourometric analyzer,b and the Access AccuTnI.c,:3,6,12,17,22 The purpose of this study was to establish reference ranges in normal dogs and cats for cTnI using a less expensive bedside analyzer, the Biosite Triage Meter®d (analyzer 4000 US dollars, each test kit 25 US dollars). The establishment of reference ranges for this machine may allow rapid and elatively inexpensive cTnI analysisr that is more readily available to veterinarians.
Materials and methods
This study was approved by the Institutional Animal Care and Use Committee at the University of Florida. Purified free canine cTnI with purity > 98% as determined by SDS-PAGEe was reconstituted with a urea-tris buffer as recommended by the manufacturer. This mixture was diluted with canine plasma (that had undetectable cTnI using the Biosite Triage Meter®d) to concentrations of 12.50 ng/mL, 3.13ng/mL, 0.78 ng/mL, 0.19 ng/ mL and 0.04 ng/mL (detection limits for this analyzer are <0.05-30 ng/mL) to assess linearity and recovery. For each dilution, the recovery of cTnI is reported (recovery = {mean cTnI value measured by the analyzer/cTnI value expected based on the calculated dilution} X 100).
Intraassay precision was assessed for the purified canine cTnI by running one test kit through the analyzer 3 times at each dilution and calculating the coefficient of variation (coefficient of variation = {standard deviation/ mean of measured values} X 100). Purified feline a Dade-Behring Diagnostics Inc, Westwood, MA, USA. b Dade-Behring, Newark, DE, USA. c Beckman Coulter Inc., Fullerton, CA, USA. d Triage Meter®; Biosite Inc, San Diego, CA, USA. e Advanced ImmunoChemical, Inc, Long Beach, CA, USA.
cTnl could not be obtained for validation of the analyzer for use in cats. Canine plasma from a clinical patient with an elevated cTnI using the Biosite Triage Meter®d was diluted to calculated concentrations of 12.05 ng/ mL, 1.21 ng/mL and 0.24 ng/mL to determine interassay precision and additional intraassay precision measurements. Interassay precision was assessed by evaluating each dilution with three test kits and intraassay precision was assessed by running one test kit through the analyzer 3 times at each dilution. The coefficient of variation was calculated for both measures of variability.
Inclusion criteria for dogs and cats entered into the study were a normal clinical history without medications (excepting heartworm prevention and flea prevention) and a normal cardiovascular evaluation based on physical examination, a 1-min, 6- lead ECG and a complete 2-dimensional, M-mode and Doppler echocardiogram. Exclusion criteria were electrocardiographic evidence of an arrhythmia (other than respiratory sinus arrhythmia) or bundle branch block, or echocardiographic evidence of congenital or acquired structural cardiac disease. Published normal values for echocardiography were used to determine if wall and chamber dimensions were normal.23-29 Specifically, wall thicknesses of <5.5 mm were considered normal for cats and wall and chamber dimensions for dogs were considered normal if they fell within the 95% prediction intervals published by Cornell et al. using allometric scaling.23,25-29 A 2 mL blood sample was drawn from the jugular or cephalic vein on the same day of the examination and placed in an EDTA tube as recommended by the manufacturer. The sample was centrifuged for 8 min at 3000 RPM and 25 °C.
The plasma was removed, separated into 3 aliquots, and frozen at -25 °C until analysis. Samples were analyzed for cardiac troponin I (cTnI) concentrations according to manufacturer’s instructions for the Biosite Triage Meter®.d,f This analyzer utilizes the fluorescence immunoassay test methodology to measure quantitative free or complexed cTnl in a 225 pL sample volume in approximately 15 min. The lower limit of detection is 0.05 ng/mL.
Simple linear regression analysis was performed on purified canine cTnl dilutions and the clinical canine cTnl dilutions using statistical software.8 Descriptive statistics and precision values are expressed as mean G standard deviation. Reference ranges are reported as medians and ranges using the 5th and 95th percentiles of the data. f Triage in Service, Your Guide to the Triage System. Biosite Inc., San Diego, CA, USA 2002:9-10. g Minitab, State College, PA, USA.
Results
Canine study
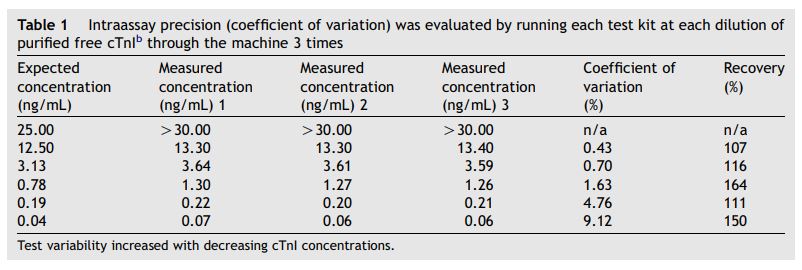
Measured values of purified free canine cardiac troponin I (cTnI) closely matched the calculated concentrations (r2 = 0.997) and results were linear across the range of dilutions used (Fig. 1). The coefficient of variation for the intraassay precision evaluated on these dilutions ranged from 0.43 to 9.12% (3.33 G 3.67%) with higher variability at the lower concentrations (Table 1). Recovery of the purified free cTnI ranged from 107-164% (Table 1).
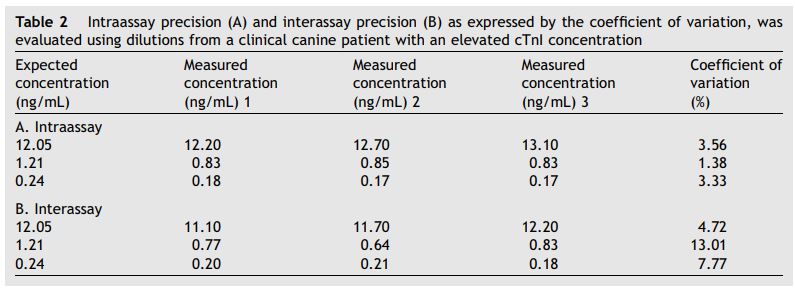
Correlations between the expected cardiac troponin I (cTnI) concentrations from the canine patient and the measured concentrations were also high (r2 = 0.997 for interassay results and r2 = 0.998 for intraassay results). Intraassay precision (coefficient of variation) ranged from 1.36 to 3.56% (2.76 G 1.20%) and interassay precision (coefficient of variation) ranged from 4.72 to 13.01% (8.5 G 4.19%) (Table 2).
Sixty-one student- and staff-owned dogs were evaluated for inclusion in the study and 55 dogs met the inclusion criteria. Six dogs were excluded due to the presence of right bundle branch block (n = 2), mild mitral regurgitation secondary to degenerative valve disease (n = 2), or premature ventricular contractions (n = 2). The 55 study dogs included 6 dogs with a grade I—II/VI left basilar systolic murmur determined to be nonpathologic based on normal echocardiographic results and 1 dog with a persistent left cranial vena cava, since this congenital vascular abnormality does not affect cardiac function.
Age was normally distributed for the population. The 55 dogs were 4.8 G 3.1 years of age and weighed 24.4 + 11.2 kg (27 castrated males, 19 spayed females, 5 males, 4 females). The population included 26 mixed breed dogs, 3 each of Boxers, Golden Retrievers, and Labrador Retrievers, 2 each of Dobermans and Staffordshire Terriers, and 1 each of Anatolian shepherd, Australian Cattle Dog, Australian shepherd, Bassett Hound, Belgian Malinois, Boston Terrier, Bulldog, Chihuahua, Cocker Spaniel, Corgi, Dogue de Bordeaux, English Springer Spaniel, German Shepard, Jack Russell Terrier, Puli, and Viszla.
Samples from 9 dogs had detectable concentrations of cardiac troponin I (cTnI). The plasma sample from 1 dog did not run due to unknown technical reasons and therefore results are for 54 dogs. The median and range (5th and 95th percentiles) for cTnI concentrations in this population of normal dogs were <0.05 ng/mL (<0.05—0.12 ng/mL).
Figure 1 Cardiac troponin I (cTnI) concentrations measured by the Biosite Triage Meter®3 compared to the expected cTnI concentration based on dilutions of purified free canine cTnI.b The line is a simple linear regression line (r2 = 0.997).
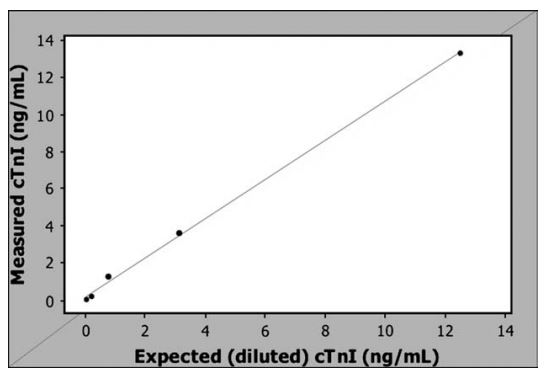
Table 1 Intraassay precision (coefficient of variation) was evaluated by running each test kit at each dilution of purified free cTnIb through the machine 3 times
Feline study
Sixty-two student- and staff-owned cats were evaluated for inclusion in the study and 58 cats met the inclusion criteria. Four cats were excluded due to the presence of left ventricular hypertrophy detected on the echocardiogram. These 58 cats included 7 cats with a grade I—II/VI sternal systolic murmur determined to be nonpathologic based on normal echocardiographic results. Ten cats with an electrocardiographic right axis deviation were included because the QRS durations were normal and the echocardiograms were normal. Age was normally distributed for the population. The 58 cats were 4.9 + 2.8 years of age and weighed 5.1 + 1.1 kg (36 castrated males, 22 spayed females). The population included 50 domestic short hairs, 4 domestic long hairs, 2 American short hairs, 1 Devon rex, and 1 Maine coon.
Samples from 3 cats had detectable concentrations of cTnI. The median cTnI concentration was <0.05 ng/mL in this population of normal cats. The range (5th and 95th percentiles) was also <0.05 because all cats between these percentiles had undetectable cTnI concentrations (only 3 cats had detectable cTnI concentrations which constituted the upper 5% of the population).
Discussion
This study established reference ranges for cardiac troponin I in clinically and cardiovascularly normal dogs and cats using the Biosite Triage Meter® .d Additionally, we performed limited validation testing for this machine for the detection of canine cTnI. Using purified, free canine cTnI, we demonstrated a high correlation between measured and expected cTnI values, showed high recovery of purified free cTnI and showed linearity across the tested dilutions. Both mean interassay variation and mean intraassay variation were low for this analyzer.
Table 2 Intraassay precision (A) and interassay precision (B) as expressed by the coefficient of variation, was evaluated using dilutions from a clinical canine patient with an elevated cTnI concentration
One limitation of this study is that we used free (not complexed to cardiac troponin C or T) canine cTnI for validation of the analyzer. The predominant circulating form of cardiac troponins in human beings is cTnI complexed to cardiac troponin C, however, the predominant circulating form in dogs is not known.19,30 The cardiac troponin forms circulating in the patient and the forms detected by analyzers both can influence the cTnI value obtained. By evaluating only free canine cardiac troponin I (cTnI), we cannot provide potentially clinically relevant information about the recovery of com- plexed forms by the Biosite Triage Meter®.d
Using the 95th percentile value from this study, the upper limit of cTnI for the normal dogs was 0.12 ng/mL and for the normal cats was <0.05 ng/ mL. Only 3 cats in this study had detectable cTnI concentrations and results from these cats were excluded from the range because they were in the top 5% of the values. After exclusion of the top 5% of canine values, 6 dogs still had detectable cTnI concentrations. Because we did not detect any clinical, electrocardiographic, or echocardio- graphic signs of cardiac disease in these dogs, because this reference range is similar other reported reference ranges for canine cardiac troponin I (cTnI), and because a low background level of cTnI can be detected in the blood due to release of cTnI from the cytosolic pool, we have interpreted these values as normal in dogs.7,15,22
Based on the results of this study, canine cTnI values above this upper limit of 0.12 ng/mL and feline cTnI values that are detectable on this analyzer may indicate cardiac injury. These cutoff values should be used with caution, however, since we did not evaluate cTnI concentrations with this analyzer in dogs or cats with cardiac disease. Additionally, we were only able to validate the analyzer for canine cTnI, as purified feline cTnI was not available. Rishniw et al., however, recently demonstrated 96% homology between canine and feline cardiac troponin I (cTnI) suggesting that cTnI analyzers validated for canine use would probably be able to detect feline cTnI.18
The reference ranges reported in this study can only be applied to cTnI results obtained from the Biosite Triage Meter®d because cardiac troponin I analyzers have not been standardized. Each manufacturer may utilize a different and proprietary target amino acid in the assay with differing abilities to detect free or complexed cTnI, often resulting in up to a 10-fold difference in the absolute values obtained between analyzers.19-21
Further study is necessary to determine cardiac troponin I (cTnI) concentrations using this analyzer in dogs and cats with cardiac disease. The affordability of the Biosite Triage Meter®d and the availability of reference ranges may make this an attractive analyzer in the veterinary setting and should further research into the role of cardiac troponin I in dogs and cats.
References
- Opie LH. Mechanisms of cardiac contraction and relaxation. In: Braunwald E, Zipes DP, Libby P, editors. Heart disease: a textbook of cardiovascular medicine. 6th ed. Philadelphia: WB Saunders; 2001. p. 444-8.
- O'Brien PJ, Landt Y, Landenson JH. Differential reactivity of cardiac and skeletal muscle from various species in cardiac troponin I immunoassay. Clin Chem 1997;43:2333-8.
- Herdon WE, Kittleson MD, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002;16:558-64.
- Connolly DJ, Cannata J, Boswood A, et al. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003;5:209-16.
- Lobetti R, Dvir E, Pearson J. Cardiac troponins in canine babesiosis. J Vet Intern Med 2002;16:63-8.
- Schober K, Cornand C, Kirbach B, et al. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilation-volvulus. J Am Vet Med Assoc 2002; 221:382-8.
- Wu AHB, Feng YJ, Moore R, et al. Characterization of cardiac troponin sub-unit release into serum after acute myocardial infarct and comparison of assays for troponin T and I. Clin Chem 1998;44:1198-208.
- DeFrancesco TC, Atkins CE, Keene BW, et al. Prospective clinical evaluation of serum cardiac troponin T in dogs admitted to veterinary teaching hospitals. J Vet Intern Med 2002;16:553-7.
- Ooi DS, Isotalo PA, Veinot JP. Correlation of anitmortem serum creatine kinase, creatine kinase-mb, troponin I, and troponin T with cardiac pathology. Clin Chem 2000;46: 338-44.
- Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac- specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996; 335:1342-9.
- Voss EM, Sharkey SW, Gernert AE, et al. Human and canine cardiac troponin T and creatine kinase-MB distribution in normal and diseased myocardium. Infarct sizing using serum profiles. Arch Pathol Lab Med 1995;119:799-806.
- Mair J. Tissue release of cardiac markers; from physiology to clinical applications. Clin Chem Lab Med 1999;37:1077-84.
- Cummins B, Cummins P. Cardiac specific troponin-I release in canine experimental myocardial infarction: development of a sensitive enzyme-linked immunoassay. Mol Cell Cardiol 1987;19:999-1010.
- Zaninotto M, Altinier S, Lachin M, et al. Strategies for the early diagnosis of acute myocardial infarction using biochemical markers. Am J Clin Pathol 1999;111:399-405.
- Oyama MA, Sisson DD. Cardiac troponin I concentration in dogs with cardiac disease. J Vet Intern Med 2004; 18: 831-9.
- Schober KE, Birbach B, Oechtering G. Noninvasive assessment of myocardial cell injury in dogs with suspected cardiac contusion. J Vet Cardiol 1999;1:17-26.
- Oyama MA, Solter PF. Validation of an immunoassay for measurement of canine cardiac troponin I. J Vet Cardiol 2004;6:17-24.
- Rishniw M, Barr SC, Simpson KW, et al. Cloning and sequencing of the canine and feline cardiac troponin I genes. Am J Vet Res 2004;65:53-8.
- KatrukhaAG, Bereznikova AV, Filatov VL, etal. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem 1998;44:2433-40.
- Saadeddin SM, Habbab MA, Siddieg HH, et al. Evaluation of 6 cardiac troponin assays in patients with acute coronary syndrome. Saudi Med J 2003;24:1092-7.
- Dasgupta A, Chow L, Nazareno L, et al. Performance evaluation of a new chemiluminescent cardiac troponin I assay. J Clin Lab Anal 2000;14:224-9.
- Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 2001;15:501-3.
- Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311-21.
- Rishniw M, Erb HN. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429-35.
- Kienle RD. Echocardiography. In: Kittleson MD, Kienle RD, editors. Small animal cardiovascular medicine. St.Louis: Mosby, Inc; 1998. p. 100-4.
- Jacobs G, Knight DH. Change in M-mode echocardiographic values in cats given ketamine. Am J Vet Res 1985;46:1712-3.
- Jacobs G, Knight DH. M-mode echocardiographic measurements in nonanesthetized healthy cats: effects of body weight, heart rate and other variables. Am J Vet Res 1985; 46:1705-11.
- Fox PR, Bond BR, Peterson ME. Echocardiographic reference values in healthy cats sedated with ketamine hydrochloride. Am J Vet Res 1985;46:1479-84.
- Dunkle N, Moise S, Scarlett-Kranz J, Short CE. Cardiac performance in cats after administration of xylazine or xylazine and glycopyrrolate: echocardiographic evaluations. Am J Vet Res 1986;47:2212-6.
- Mockel M, Heller G, Berg K, et al. The acute coronary syndrome diagnosis and prognostic evaluation by troponin I is influenced by the test system affinity to different troponin complexes. Clin Chim Acta 2000;293:139-55.
^Наверх









