An update on canine cardiomyopathies - is it all in the genes?

Author information
Dutton E., López-Alvarez J. An update on canine cardiomyopathies - is it all in the genes? // J Small Anim Pract. 2018 Apr 17. doi: 10.1111/jsap.12841. [Epub ahead of print]
Abstract
Dilated cardiomyopathy is the second most common cardiac disease in dogs and causes considerable morbidity and mortality. Primary dilated cardiomyopathy is suspected to be familial, and genetic loci have been associated with the disease in a number of breeds. Because it is an adult-onset disease, usually with late onset, testing breeding dogs and bitches before breeding for a genetic mutation that could lead to dilated cardiomyopathy would be helpful to prevent disease. There is growing evidence that the genetic basis may be multigenic rather than monogenic in the majority of studied breeds. This review article describes the known genetic aspects of canine dilated cardiomyopathy and the implications of genetic tests on heart testing and the future of veterinary cardiology.
INTRODUCTION
Dilated cardiomyopathy (DCM) is the second most common form of acquired cardiac disease in the dog after degenerative mitral valve disease and the most prevalent in large and giant breeds of dogs. Breeds reported as having the highest prevalence of DCM include Dobermanns, boxers, great Danes, Newfoundlands, Irish wolfhounds (IWH) and English cocker spaniels (Monnet et al. 1995, Borgarelli et al. 2006, Martin et al. 2009).
Other breeds with a less common diagnosis of DCM mentioned in these studies are: German shepherd dog, Saint Bernard, Labrador and golden retriever, Neapolitan mastiff, Rottweiler, dogue de Bordeaux, Leonberger, collie, Dalmatian, greyhound and mixed breed. DCM has been classically defined as a primary myocardial disorder causing systolic dysfunction with secondary ventricular dilation (Fig 1), normal to reduced wall thickness and increased cardiac mass resulting from myocyte enlargement (Maron et al. 2006). However, this definition is limited to a morphological diagnosis and does not include any of the electrocardiographic abnormalities that accompany the disease.
For this reason, we prefer referring to Dilated cardiomyopathy as a primary syndromic disease of the myocardium causing either mechanical dysfunction, leading to dilation and congestion, or electrical dysfunction, leading to arrhythmias and sudden death, or both mechanical and electrocardiographic abnormalities together. Indeed, DCM can be considered a syndrome causing either signs associated with congestive heart failure (pulmonary oedema, cavitary effusions) or forward heart failure signs (exercise intolerance, mucous membrane pallor, syncope) and, often, both.
Many systemic diseases can lead to a dilated, poorly contractile heart, mimicking the DCM phenotype. Examples include chronic volume overload, hypothyroidism, myocarditis and nutritional and environmental factors such as taurine deficiency and anthracycline toxicity. Tachycardia-induced cardiomyopathy (TICM) is also a cause of secondary DCM in the dog, being relatively frequent in certain breeds, such as the Labrador retriever (Perego et al. 2012). It is necessary to actively rule out these underlying causes of systolic dysfunction when diagnosing DCM, explaining why many authors state that DCM is a diagnosis of exclusion (Dukes-McEwan et al. 2003). Primary Dilated cardiomyopathy remains an idiopathic disease, but there is growing evidence that, as in humans, canine DCM has a strong genetic basis with marked familial transmission. However, as in people, the exact molecular biology mechanisms leading to its phenotypic expression are still unknown. It has been hypothesised that each breed may have its own particular genetic mutation leading to DCM. It is possible that various dog breeds share the same or similar causative genetic disorders.
It is also possible that, within a single dog breed, there may be more than one genetic abnormality causing this syndrome. However, given the inbreeding practices and relative genetic homogeneity, the latter appears less likely In people, more than 50 loci have been associated with the suspected monogenic form of DCM, but the mutations located in these DCM-related genes account for only approximately 50% of human cases of Dilated cardiomyopathy (Posafalvi et al. 2013). The other half is thought to be associated with multiple synergistic mutations or due to poorly understood genetic interactions (McNally et al. 2013).
Furthermore, many authors believe that, as with many other genetic diseases, DCM has an incomplete penetrance (Meurs et al. 2012); i.e., not all dogs with the same disease-causing mutation will develop the disease. Similarly, it has also been stated that dilated cardiomyopathy may have variable expression, referring to the wide spectrum of clinical signs and abnormalities that occur in dogs with DCM (Meurs et al. 2012): the same mutation may not affect all patients with the same intensity. The phenomena of incomplete penetrance and variable expression probably result from the interaction between the genetic information and the differential activation of their regulatory epigenetic factors. Different lifestyles, diet and other environmental factors may alter the way the genetic code determines not only every organ’s normal physiology in each dog but also the diseases affecting them. The two main histological findings that have been described in canine cardiomyopathies include attenuated wavy fibres and fibro-fatty infiltration of the myocardium (Tidholm & Jönsson 1997).
Atrophy, attenuation and the wavy appearance of cardiomyocytes (generally surrounded by oedema) appears to be the common response of cardiac cells to various pathological stimuli. As a consequence, the presence of attenuated wavy fibres is reported to be a highly sensitive (98%) finding for DCM diagnosis in dogs (Tidholm & Jönsson 2005). The histological features seen with boxers with arrhythmogenic right ventricular cardiomyopathy (ARVC) and some Dobermanns with dilated cardiomyopathy are myocytolysis, myofibre degeneration, vacuolisation and myocyte atrophy with extensive fibrosis and fatty infiltration. Fibrofatty infiltration provides the arrhythmogenic substrate in these breeds, and possibly others, such as the great Dane (Fig 2a, b). The natural history and progression of dilated cardiomyopathy is not well described for all predisposed breeds, but in general, it is possible to distinguish two phases by the presence or absence of clinical signs (Wess et al. 2017). The preclinical phase is often a long asymptomatic period, detected only during screening programmes, careful clinical examination or preanaesthetic evaluations. The second phase is called the clinical or overt phase because clinical signs are evident. There is a wealth of differences between the predisposed breeds regarding their survival time from diagnosis, clinical manifestations, histopathology changes, inheritance patterns and age of onset.
FIG 1. Echocardiographic image from a deerhound with preclinical dilated cardiomyopathy acquired from a right parasternal long axis four-chambers view. There is a dilated, rounded left ventricle.
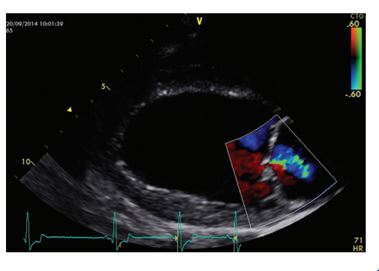
Colour flow shows a mild central jet of mitral regurgitation that occurs secondary to dilation of the mitral annulus fibro-fatty infiltration of the myocardium (Tidholm & Jönsson 1997).
Atrophy, attenuation and the wavy appearance of cardiomyocytes (generally surrounded by oedema) appears to be the common response of cardiac cells to various pathological stimuli. As a consequence, the presence of attenuated wavy fibres is reported to be a highly sensitive (98%) finding for DCM diagnosis in dogs (Tidholm & Jönsson 2005). The histological features seen with boxers with arrhythmogenic right ventricular cardiomyopathy (ARVC) and some Dobermanns with dilated cardiomyopathy are myocytolysis, myofibre degeneration, vacuolisation and myocyte atrophy with extensive fibrosis and fatty infiltration. Fibrofatty infiltration provides the arrhythmogenic substrate in these breeds, and possibly others, such as the great Dane (Fig 2a, b).
The natural history and progression of dilated cardiomyopathy is not well described for all predisposed breeds, but in general, it is possible to distinguish two phases by the presence or absence of clinical signs (Wess et al. 2017). The preclinical phase is often a long asymptomatic period, detected only during screening programmes, careful clinical examination or preanaesthetic evaluations. The second phase is called the clinical or overt phase because clinical signs are evident. There is a wealth of differences between the predisposed breeds regarding their survival time from diagnosis, clinical manifestations, histopathology changes, inheritance patterns and age of onset. DOBERMANN DCM is a highly prevalent disease in Dobermanns, which explains why this breed is often used as a model for the disease in dogs. The cumulative prevalence of DCM in Dobermann in Europe has been reported as being 58% (Wess et al. 2010). As DCM can occur in Dobermann between 2 and 4 years of age with a prevalence of 10%, screening is advised starting from 3 to 4 years of age (Wess et al. 2017). Morphological changes (i.e. left ventricular dilation) and electrical changes (either ventricular or atrial arrhythmias) have been described in Dobermanns with dilated cardiomyopathy and may occur together or separately. Therefore, complete screening should include a 24-hour ambulatory electrocardiogram (ECG) evaluation (Holter monitor) and echocardiography.
FIG 2. Haematoxylin and eosin stain from the myocardium of a great dane diagnosed with dilated cardiomyopathy.
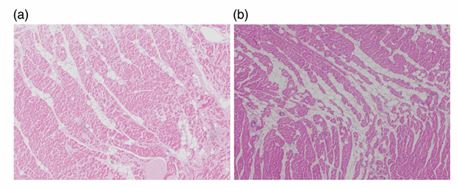
This shows areas of focal interstitial fibrosis, adipose tissue infiltration and mild myocardial degeneration on the left ventricle (a) and the right ventricle (b).
DCM was previously regarded as affecting male Dobermanns more than females (Calvert et al. 1997a), but a more recent study shows no overall difference in the occurrence of cardiomyopathy between male and female dogs. However, there is a difference between disease manifestations depending on the gender of the dog (Wess et al. 2010): male Dobermanns are more likely to develop early echocardiographic changes, whereas females are more likely to develop ventricular arrhythmias. Sudden death is a concern in the Dobermann, occurring in a third of affected dogs during the preclinical phase (Calvert et al. 1997b) and in a third during the overt phase (Calvert et al. 1997a).
Sudden death is presumed to be due to ventricular tachycardia (Fig 3) leading to ventricular fibrillation and subsequent lack of cardiac output (Calvert et al. 1997b). Due to the high prevalence of both hypothyroidism and dilated cardiomyopathy in some breeds, especially the Dobermann, there has been some confusion about the role of this endocrine disease in causing dilated cardiomyopathy. Although severe and chronic hypothyroidism may cause marked systolic dysfunction (potentially leading to congestive heart failure), it is now clear that, epidemiologically, hypothyroidism is not the primary cause of dilated cardiomyopathy in this breed (Calvert et al. 1998, Beier et al. 2015). DCM is thought to be an autosomal dominant trait in the Dobermann (Meurs et al. 2007). Ten cardiac genes known to be associated with familial DCM in human patients have been evaluated in Dobermanns, but none of the coding regions of genes associated with DCM in humans appear to consistently account for dilated cardiomyopathy in this breed (O’Sullivan et al. 2011). A genome-wide association study on 141 Dobermanns from Germany and validated in an independent cohort of dogs from the UK showed that a locus on chromosome 5 contains a DCM-associated single nucleotide polymorphism (SNP), “C-allele” (Mausberg et al. 2011). The dogs with isolated arrhythmias (as opposed to the “dilation” group) had the highest frequency of the C-allele, but it was not possible to associate a candidate gene for DCM under this association signal. A study carried out on an American population of Dobermanns showed that a splice site mutation in a gene encoding for pyruvate dehydrogenase kinase 4 (PDK4), a mitochondrial protein, on chromosome 14 was associated with the development of DCM in the Dobermann (Meurs et al. 2012).
However, a later study, on a population of Dobermanns from Europe, had remarkably different results, with no association found between DCM and the PDK4 mutation (Owczarek-Lipska et al. 2013). This difference in results may have been due to different genetic populations within America compared with Europe. Mitochondrial dysfunction resulting from the PDK4 mutation was studied further in a small number of American Dobermanns (Sosa et al. 2016). Acetyl coenzyme A is the molecule from which energy can be obtained in the mitochondria, and the sources of this molecule are mainly carbohydrates, fatty acids and amino acids.
The choice of pathway between glycolysis and fatty acid oxidation is regulated by the pyruvate dehydrogenase complex (the function of which is inhibited by PDK4) as well as other factors such as energy starvation. The preferred cardiac energy source in the healthy heart is fatty acids (Grynberg & Demaison 1996).
FIG 3. This electrocardiogram from a Dobermann shows a wide complex ventricular tachycardia (50mm/s and 10 mm/mV)
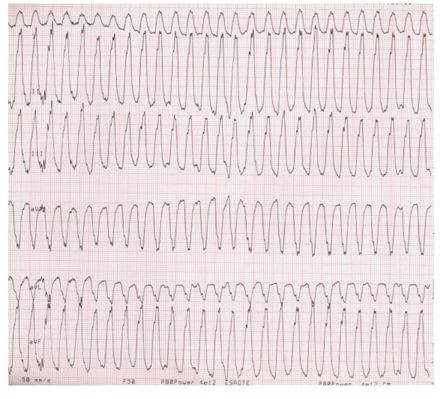
It was presumed that, in Dobermanns with the PDK4 mutation, reduction of PDK4 expression would lead to deficient control of the pyruvate dehydrogenase complex, shifting metabolism away from fatty acid oxidation and increasing glycolysis (Sosa et al. 2016). Over time, unregulated glycolysis could potentially lead to impaired mitochondrial electron transport and predispose cells to mitochondrial damage, as observed in Dobermans with DCM and the PDK4 mutation (Meurs et al. 2012). The results of the American study indicated that Dobermanns with dilated cardiomyopathy and the PDK4 mutation had a lower basal mitochondrial oxygen consumption rate than did healthy Dobermanns , suggesting mitochondrial dysfunction (Sosa et al. 2016). Nevertheless, and especially in view of the small sample size, the significance of the PDK4 mutation as a dilated cardiomyopathy risk factor remains uncertain (Sosa et al. 2016).
A test for a second mutation has recently been commercialised in this breed. However, to our knowledge, the research supporting this commercial test has not been published in a peerreviewed journal. A meta-analysis of the available canine DCM genetic datasets showed there was an interaction between the known dilated cardiomyopathy loci (on chromosome 5 and PDK4) and an unknown X-linked locus in Dobermanns (Simpson et al. 2015). The study evaluated the potential multigenic contribution to the disease rather than treating it as monogenic and suggested a possible genetic basis for the known gender differences in affected Dobermanns .
Finally, microRNA (miRNA) are small, non-coding RNA molecules that are highly conserved across species. MiRNAs regulate each aspect of the translation of the genetic information and, therefore, are thought to participate as determinant epigenetic factors both in health and disease. An underpowered study evaluating circulating miRNA expression in German Dobermanns showed a non-significant trend in expression patterns in affected dogs (Steudemann et al. 2013). The study concluded that the technique for the evaluation of miRNAs must be improved. However, it opens up a promising new research horizon for the investigation of genetic diseases and, more specifically, for the investigation of the regulation of the genetic code transcription for DCM, regardless of whether it is a mono- or a polygenic disease. BOXER Boxers have a particular form of adult-onset cardiomyopathy that does not correspond with the classical description of DCM. “Boxer cardiomyopathy” was the name first given and was described by Harpster in 1983. He classified the disease into three categories that were thought to occur in equal proportions: (1) the preclinical phase with arrhythmias but no clinical signs, (2) the overt phase with episodic syncope during exercise or excitement and (3) the myocardial dysfunction phase with congestive heart failure and arrhythmias. Extensive myocardial histological changes consistent with myocyte atrophy, patchy necrosis and fibro-fatty infiltration affecting most extensively the right ventricle, with no (or little) left ventricular dilation, was noted.
This original description is still applicable to both European and American boxers, and some authors have suggested a chronological progression from one phase to the next. In 1999, “boxer cardiomyopathy” was compared with its homologous disease in humans, known as ARVC (arrhythmogenic right ventricular cardiomyopathy) (Wotton 1999). It was described then that boxers with ARVC have a strong familial predisposition to suffer from ventricular premature complexes (VPC) with left bundle branch block morphology, suggestive of right ventricular origin. Other ECG conduction abnormalities included notched QRS complexes and various forms of first- and second-degree atrioventricular block as well as left bundle branch block. This study described similar pathology and histology changes as described by Harpster (1983). Evaluation of boxer families shows that ARVC is inherited as an autosomal dominant trait with age-related penetrance (Meurs et al. 1999). In collaboration with human ARVC researchers, convincing evidence was provided regarding the similarities with its human counterpart by producing a thorough pathological description of the disease (Basso et al. 2004). However, the genetics of the disease are still not fully understood. More than 20 loci have been described in human ARVC, often associated with desmosomal proteins of the intercalated disc.
In people, abnormal intercalated disc conformations may lead to an increased susceptibility to environmental agents causing myocarditis, cardiomyocyte death, fibro-fatty replacement and, ultimately, ARVC (Fontaine et al. 1999, Calabrese et al. 2006). In boxers, the cardiac ryanodine receptor (RyR2) protein and its messenger RNA expression were found to be reduced in those with ARVC. However, no genetic association with the RyR2 gene was demonstrated in this breed (Meurs et al. 2006). In addition, calstabin-2 messenger RNA expression was shown to be significantly down-regulated in the myocardium from ARVC boxers compared with healthy controls and Dobermanns with DCM. This was thought to be a potential mechanism related to the occurrence of ventricular arrhythmias in boxers with ARVC, but no causative mutations were found (Oyama et al. 2008).
The use of immunofluorescence labelling of proteins has allowed the evaluation of the molecular composition of the intercalated disc in boxers with ARVC (Oxford et al. 2007). This showed that ARVC causes loss of molecular integrity of the intercalated disc as a whole, loss of gap junction plaques and remodelling of other intercalated disc structures. However, no mutations were found to affect the desmosomal proteins evaluated (desmoplakin, plakoglobin, connexin-43 and plakophilin-2). The abnormalities found on the proteins from the intercalated disc were possibly more of a consequence rather than the cause of the disease. Moreover, boxers with ARVC had fewer desmosomes, adherens junctions and gap junctions compared with healthy boxers (Oxford et al. 2011). In addition, the ARVC boxers had abnormalities in sarcomeric structure, suggesting a novel link between ARVC and the actin-myosin contractile apparatus. Both abnormalities could result in loss of cell-to-cell attachment and decreased electrical and mechanical communication between cardiac myocytes.
Finally, a genome-wide association study revealed a significant association between an 8-base pair deletion on chromosome 17 in American boxers with ARVC compared with healthy boxers (Meurs et al. 2010). This mutation was associated with a reduction of messenger RNA production for the protein striatin. Immunofluorescence localised striatin to the intercalated disc region of cardiomyocytes, co-localising with the proteins desmoplakin, plakoglobin and plakophilin-2. In this study, the striatin mutation was identified in 57 of 61 ARVC boxers (either as homozygotes or heterozygotes) but was also present in nine of 38 unaffected control boxers (all heterozygotes). Boxers that were homozygous had significantly more arrhythmias than heterozygous or wild-type boxers. Moreover, when a well-characterised population of boxers from the UK was analysed, it was found that the striatin mutation was extremely common (Dukes-McEwan et al. 2010, Cattanach et al. 2015).
The striatin mutation did not explain the occurrence of ARVC in UK boxers because no significant difference in proportions of genotypes between affected and healthy dogs were found. These and further discrepancies about the striatin mutation have generated some discussion (Meurs et al. 2014, Oxford et al. 2014). To investigate this conundrum, a thorough pedigree analysis of ARVC boxers from the UK showed that affected UK individuals originated from the same American lines described by Harpster (Cattanach et al. 2015). The common genetic background with differing striatin results was considered sufficient to dismiss the striatin mutation as the only cause for ARVC. The study concluded that the causative mutation was very likely to co-localise with the striatin gene on chromosome 17. However, this same study recognised that boxers homozygote for the striatin mutation tended to be severely affected at early ages. This suggested that the striatin mutation may have indeed had a modulating effect on the cardiomyocyte, but it was not the aetiological agent for ARVC in boxers (Cattanach et al. 2015).
Lastly, it does appear that there are marked geographical differences in the occurrence of ARVC within Europe (and probably America) (personal communication). There are geographical areas where veterinary cardiologists have rarely seen boxers with right ventricular ectopic activity as a main feature of their cardiac disease. However, although the prevalence of the disease may be much lower in these regions, cases of myocardial dysfunction in boxers are also described. Therefore, it is possible that there is more than one myocardial disease affecting this breed, but it could also be that the “ARVC phenotype” is diverse.
IRISH WOLFHOUND
The prevalence of dilated cardiomyopathy in the IWH is reported as being between 24.2 and 29% (Vollmar 2000, Distl et al. 2007, Vollmar & Fox 2016). The onset of disease is between 3 and 7 years, with a mean of 4•52±1•99 years. DCM is more prevalent in male IWHs than females, and males are often affected at a younger age than females (Vollmar 2000, Simpson et al. 2016).
An association has been shown between the presence of atrial fibrillation (AF) and the development of DCM in the IWH, with AF being a potential precursor to secondary DCM (Vollmar 2000, Vollmar & Fox 2016). However, not all IWHs diagnosed with AF go on to develop DCM, and some IWHs diagnosed with DCM do not have AF (Simpson et al. 2016). Reports suggest that between 80.5 and 87.6% of individuals diagnosed with DCM are concurrently diagnosed with AF, which provides evidence that IWHs diagnosed with AF should be closely monitored to ensure that they do not go on to develop dilated cardiomyopathy (Vollmar 2000, Simpson et al. 2016). Diagnosing DCM early in the disease process is important as there is evidence that treatment in the preclinical stage of DCM prolongs the period before onset of heart failure and extends survival (Summerfield et al. 2012, Vollmar & Fox 2016).
A gender-dependent dominant gene model is thought to play a major role in the expression of DCM in the IWH, but a monogenic model alone cannot explain the occurrence of this disease (Distl et al. 2007). As DCM has a higher prevalence in male than female IWHs, one study examined the role of tafazzin, a protein produced by an X chromosome-related gene (TAZ gene), which is highly expressed in the myocardium (Philipp et al. 2007). The TAZ gene was not shown to influence the development of DCM in the IWH (Philipp et al. 2007). Using the genome-wide association technique, two studies showed that multiple loci had significant associations with the development of DCM in the IWH (Philipp et al. 2012, Simpson et al. 2016).
The results of these two studies were different in that only three of the five SNPs associated with DCM in the European IWHs were associated with dilated cardiomyopathy in the UK study. Of these three SNPs, on chromosomes 1, 21 and 37, only one had the same allele associated with disease. These differences may have been due to different genetic backgrounds for both populations or due to difficulties in correctly classifying affected and control dogs. Most importantly, both studies concluded that an oligogenic inheritance of DCM is most likely to occur in IWHs and that additional unidentified genetic factors may be involved. This conclusion was derived from the fact that certain loci were associated with the disease in some individuals but not at a population level. However, when all three loci were combined, the result was the identification of a genotype that conferred a greater risk of disease (Simpson et al. 2016).
NEWFOUNDLAND
The prevalence of dilated cardiomyopathy in the Newfoundland has been estimated to be 10% (Dukes-McEwan 1999). Newfoundlands have the fifth highest death rate from heart disease of all breeds according to a Swedish survey of insured pedigree dogs (Egenvall et al. 2006). The median age of onset of clinical signs in Newfoundlands with DCM is 8 years of age, and the median age of death is 9 years (Dukes-McEwan 2000). It is thought that disease progression might be slower in the Newfoundland compared with other breeds of dog.
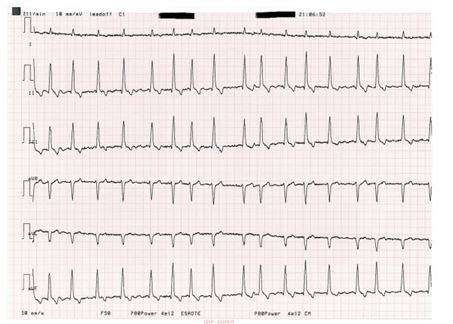
The reported median survival time in dogs of all breeds diagnosed with mainly overt dilated cardiomyopathy was 19weeks (Martin et al. 2009) compared with 6 months for Newfoundlands (Tidholm & Jönsson 1996). The classical progression of dilated cardiomyopathy in this breed has been described as progressive reduction in contractility followed by enlargement of the cardiac chambers, eventually leading to the development of AF (Fig 4) and congestive heart failure. Up to 77% of Newfoundlands with DCM had AF in a retrospective study (Martin et al. 2009).
Echocardiographic studies show that Newfoundlands with left ventricular enlargement alone progress more rapidly (within 2 years) than those with depressed fractional shortening alone, which deteriorate over several years (Dukes-McEwan et al. 2003). One study demonstrated predisposition to dilated cardiomyopathy in an extended family of Newfoundlands from the UK (Dukes-McEwan & Jackson 2002). Pedigree analysis showed an autosomaldominant mode of inheritance with incomplete penetrance, although an autosomal-recessive transmission could not be excluded. A low-resolution genome scan of 48 dogs using over 200 markers showed minimal polymorphism and limited heterozygosity.
A later study, using the same families of Newfoundlands plus further extended families, excluded 15 candidate genes as a cause of DCM in 74 Newfoundlands (Wiersma et al. 2008). Thirty-eight dogs were affected with DCM, and 36 were used as healthy controls. The conclusions of the study were that further studies, including a genome scan with a marker set of higher resolution and a larger set of samples, were warranted. Another study was equally unsuccessful at determining the genetic origin of the disease in this breed (Davidsson 2007). Finally, plasma taurine concentration (a surrogate marker of myocardial taurine concentration) measured in 216 Newfoundlands showed that taurine deficiency occurred in 8% of dogs (Backus et al. 2003).
Taurine is an important amino acid for myocardial function. It can be synthesised from dietary cysteine and methionine in the dog; hence, it is not considered essential in the dog. In this study, taurine-deficient Newfoundlands were older, less active and had more medical problems. Some of the taurine-deficient dogs had secondary dilated cardiomyopathy, which could be reversed after taurine supplementation. It was suggested that taurine synthesis was inadequate to meet taurine requirements in this breed. It was hypothesised that some breeds of dogs may have increased taurine loss or may have different taurine biosynthetic rates, making them more sensitive to diets with marginal taurine concentration. This shows that, in at least a proportion of Newfoundlands with poorly contractile hearts, the cardiac changes may be secondary to dietary taurine deficiency rather than due to idiopathic DCM.
GREAT DANE
The prevalence of dilated cardiomyopathy in the great Dane varies from 3.9% in referral settings (Sisson & Thomas 1995) to between 11.8 and 35.6% in prospective screening studies (Tarducci et al. 2003, Stephenson et al. 2012). The natural history, inheritance and disease progression of dilated cardiomyopathy in the great Dane has scarcely been studied. DCM in this breed is suspected to have a long preclinical phase.
However, once the disease becomes apparent, great Danes may have the shortest median survival times compared with other breeds (Martin et al. 2010). It was originally thought that the most common arrhythmia in great Danes was AF secondary to atrial dilation in the congestive phase of the disease (Meurs et al. 2001). This concept was challenged when it was found that unreported sudden death was occurring in a large proportion of sires and dams at early ages. A prospective screening study in the UK found that the proportion of dogs with AF was rather low, whereas ventricular arrhythmias were present in 30% of the dogs screened, and in 56% of great Danes with clinical dilated cardiomyopathy (Stephenson et al. 2012).
FIG 4. This electrocardiogram from a Newfoundland dog with dilated cardiomyopathy shows a fast rate (heart rate of more than 180bpm) with irregular rhythm and no discernible P waves on any limb lead. This is consistent with atrial fibrillation (50mm/s and 10 mm/mV)
An American pedigree analysis of 17 great Danes with DCM suggested that the disease may be transmitted as a recessive trait linked to the gender-determining chromosome X (Meurs et al. 2001). This study concluded that affected dogs should not be used for breeding – particularly affected females – because their male offspring were at an increased risk of suffering from the disease.
A larger pedigree analysis from the UK included 107 dogs (Stephenson et al. 2012) and found that the mode of inheritance was most consistent with an autosomal dominant trait, although polygenic inheritance could not be completely excluded. To our knowledge, evaluation of possible genetic mutations has not been published for the great Dane. One study evaluated the presence of polymorphic markers within 14 published candidate genes for dilated cardiomyopathy in the breed (Wiersma et al. 2007), but no possible causative mutations were identified.
COCKER SPANIELS
Cocker spaniels have been named with relative frequency as the one small breed that suffers from DCM. The first report analysing data from 49 English cocker spaniels from a kennel with familial history of cardiac disease determined that this breed suffers from a cardiomyopathy (Gooding et al. 1982). Later, eight cases of young English cocker spaniels with severe congestive heart failure were reviewed and diagnosed with dilated cardiomyopathy (Thomas 1987). Moreover, some English cocker spaniels with DCM and congestive heart failure have pulsus alternans at the time of diagnosis (Moneva-Jordan et al. 2007).
However, despite advanced cardiomyopathy cases being described and terminal congestive heart failure occurring in these dogs, cocker spaniels have long survival times: at the end of a small 4-year clinical trial, six of 10 cocker spaniels were still alive, and three of 10 had died of noncardiac disease (Luis-Fuentes et al. 2002). No genetic studies have been reported in this breed. In fact, it is not completely clear whether cocker dilated cardiomyopathy is a hereditary condition or whether it is secondary to a dietary deficiency of taurine and carnitine. It is also possible that there are differences between English and American cockers in that regard. In a study evaluating plasma taurine levels in dogs with DCM from different breeds, it was shown that concentration was low in 13 of 75 dogs (17%) (Kramer et al. 1995).
This deficiency occurred in certain breeds, such as American cocker spaniels and golden retrievers, but plasma taurine concentration in breeds more commonly affected with dilated cardiomyopathy was within the reference range. A small clinical trial including 11 American cocker spaniels with dilated cardiomyopathy showed that all dogs were found to have low plasma taurine concentrations at baseline.
References
- Alroy, J., Rush, J. E., Freeman, L., et al. (2000) Inherited infantile dilated cardiomyopahy in dogs: genetic, clinical, biochemical, and morphologic findings. American Journal of Medical Genetics 95, 57-66
- Alroy, J., Rush, J. E. & Sarkar, S. (2005) Infantile dilated cardiomyopathy in Portuguese water dogs: correlation of the autosomal recessive trait with low plasma taurine at infancy. Amino Acids 28, 51-56
- Backus, R. C., Cohen, G., Pion, P. D., et al. (2003) Taurine deficiency in Newfoundlands fed commercially available complete and balanced diets. Journal of the American Veterinary Medical Association 223, 1130-1136
- Basso, C., Fox, P. R., Meurs, K. M., et al. (2004) Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs. A new animal model of human disease. Circulation 109, 1180-1185
- Beier, P., Reese, S., Holler, P. J., et al. (2015) The role of hypothyroidism in the etiology and progression of dilated cardiomyopathy in Doberman pinschers. Journal of Veterinary Internal Medicine 29, 141-149
- Birkegård, A. C., Reimann, M. J., Martinussen, T., et al. (2016) Breeding restrictions decrease the prevalence of myxomatous mitral valve disease in cavalier king Charles spaniels over an 8- to 10-year period. Journal of Veterinary Internal Medicine 30, 63-68
- Borgarelli, M., Santilli, R. A. & Chiavegato, D. (2006) Prognostic indicators for dogs with dilated cardiomyopathy. Journal of Veterinary Internal Medicine 20, 104-110
- Calabrese, F., Basso, C., Carturan, E., et al. (2006) Arrhythmogenic right ventricular cardiomyopathy/dysplasia: is there a role for viruses? Cardiovascular Pathology 15, 11-17
- Calvert, C. A., Pickus, C. W., Jacobs, G. J., et al. (1997a) Signalment, survival, and prognostic factors in Doberman pinschers with end-stage cardiomyopathy. Journal of Veterinary Internal Medicine 11, 323-326
- Calvert, C. A., Hall, G. & Jacobs, G. (1997b) Clinical and pathologic findings in Doberman pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure: 54 cases (1984-1991). Journal of the American Veterinary Medical Association 15(210), 505-511
- Calvert, C. A., Jacobs, G., Medleau, L., et al. (1998) Thyroid-stimulating hormone stimulation tests in cardiomyopathic Doberman pinschers: a retrospective study. Journal of Veterinary Internal Medicine 12, 343-348
- Cattanach, B. M., Dukes-McEwan, J., Wotton, P. R., et al. (2015) A pedigree-based genetic appraisal of boxer ARVC and the role of the Striatin mutation. Veterinary Record 176, 492
- Davidsson, K. (2007) Evaluation of Genomic DNA from Paraffin Embedded Tissue and Desmin as Candidate Gene for Dilated Cardiomyopathy in Newfoundland dogs. PhD Thesis. Dept. of Animal Breeding and Genetics, Uppsala, Sweden.
- Distl, O., Vollmar, A. C., Broschk, C., et al. (2007) Complex segregation analysis of dilated cardiomyopathy (DCM) in Irish wolfhounds. Heredity 99, 460-465
- Dukes-McEwan, J. (1999) Echocardiographic/Doppler Criteria of Normality, the Findings of Cardiac Disease and the Genetics of Familial Dilated Cardiomyopathy in Newfoundlands. PhD Thesis. Royal (Dick) School of Veterinary Studies. The University of Edinburgh. Edinburgh, U.K. pp 108-116.
- Dukes-McEwan, J. (2000) Dilated cardiomyopathy (DCM) in Newfoundland dogs. Proceedings of the American College of Veterinary Internal Medicine Forum; May 24-27. American College of Veterinary Internal Medicine, Seattle, WA, USA/ Lakewood, CO, USA. pp 118-119.
- Dukes-McEwan, J. & Jackson, I. J. (2002) The promises and problems of linkage analysis by using the current canine genome map. Mammalian Genome 13, 667-672
- Dukes-McEwan, J., Borgarelli, M., Tidholm, A., et al. (2003) Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. Journal of Veterinary Cardiology 5, 7-19
- Dukes-McEwan, J., Stephenson, H., Wotton, P. R., et al. (2010) Cardiomyopathy in boxer dogs. Veterinary Times 40, 6-9
- Egenvall, A., Bonnett, B. N. & Haggström, J. (2006) Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. Journal of Veterinary Internal Medicine 20, 894-903
- Fontaine, G., Fontaliran, F., Herbert, J., et al. (1999) Arrhythmogenic right ventricular dysplasia. Annual Review of Medicine 50, 17-35
- Gooding, J. P., Robinson, W. F., Wyburn, R. S., et al. (1982) A cardiomyopathy in the English cocker spaniel: a clinico-pathological investigation. Journal of Small Animal Practice 23, 133-149
- Grynberg, A. & Demaison, L. (1996) Fatty acid oxidation in the heart. Journal of Cardiovascular Pharmacology 28 (Suppl 1), S11-S17
- Harpster, N. (1983) Boxer cardiomyopathy. In: Current Veterinary Therapy VIII. Ed R. Kirk. WB Saunders, Philadelphia, PA, USA. pp 329-337
- Kittleson, M. D., Keene, B., Pion, P. D., et al. (1997) Results of the multicenter spaniel trial (MUST): taurine and carnitine-responsive dilated cardiomyopathy in American cocker spaniels with decreased plasma taurine concentration. Journal of Veterinary Internal Medicine 11, 204-211
- Kramer, G. A., Kittleson, M. D. & Fox, P. R. (1995) Plasma taurine concentration in normal dogs and dogs with heart disease. Journal of Veterinary Internal Medicine 9, 253-258
- Luis-Fuentes, V., Corcoran, B., French, A., et al. (2002) A double-blind, randomized, placebo-controlled study of pimobendan in dogs with dilated cardiomyopathy. Journal of Veterinary Internal Medicine 16, 255-261
- Maron, B. J., Towbin, J. A., Thiene, G., et al. (2006) Contemporary definitions and classification of the cardiomyopathies. Circulation 113, 1807-1816
- Martin, M. W., Stafford Johnson, M. J. & Celona, B. (2009) Canine dilated cardiomyopathy: a retrospective study of signalment, presentation and clinical findings in 369 cases. Journal of Small Animal Practice 50, 23-29
- Martin, M. W., Stafford Johnson, M. J., Strehlau, G., et al. (2010) Canine dilated cardiomyopathy: a retrospective study of prognostic findings in 367 clinical cases. Journal of Small Animal Practice 51, 428-436
- Mausberg, T. B., Wess, G., Simak, J., et al. (2011) A locus on chromosome 5 is associated with dilated cardiomyopathy in doberman pinschers. PLoS One 6, 5-10
- McNally, E. M., Golbus, J. R. & Puckelwartz, M. J. (2013) Genetic mutations and mechanisms in dilated cardiomyopathy. The Journal of Clinical Investigation 123, 19-26
- Meurs, K. M., Spier, A. W., Miller, M. W., et al. (1999) Familial ventricular arrhythmias in boxers. Journal of Veterinary Internal Medicine 14, 437-439
- Meurs, K. M., Miller, M. W. & Wright, N. A. (2001) Clinical features of dilated cardiomyopathy in great Danes and results of a pedigree analysis: 17 cases (1990- 2000). Journal of the American Veterinary Medical Association 218, 729-732
- Meurs, K. M., Lacombe, V. A., Dryburgh, K., et al. (2006) Differential expression of the cardiac ryanodine receptor in normal and arrhythmogenic right ventricular cardiomyopathy canine hearts. Human Genetics 120, 111-118
- Meurs, K. M., Fox, P. R., Norgard, M., et al. (2007) A prospective genetic evaluation of familial dilated cardiomyopathy in the Doberman pinscher. Journal of Veterinary Internal Medicine 21, 1016-1020
- Meurs, K. M., Mauceli, E., Lahmers, S., et al. (2010) Genome-wide association identifies a deletion in the 3′ untranslated region of Striatin in a canine model of arrhythmogenic right ventricular cardiomyopathy. Human Genetics 128, 315- 324
- Meurs, K. M., Lahmers, S., Keene, B. W., et al. (2012) A splice site mutation in a gene encoding for PDK4, a mitochondrial protein, is associated with the development of dilated cardiomyopathy in the Doberman pinscher. Human Genetics 131, 1319-1325
- Meurs, K. M., Stern, J. A., Reina-Doreste, Y., et al. (2014) Natural history of arrhythmogenic right ventricular cardiomyopathy in the boxer dog: a prospective study. Journal of Veterinary Internal Medicine 28, 1214-1220
- Moneva-Jordan, A., Luis-Fuentes, V., Corcoran, B. M., et al. (2007) Pulsus alternans in English cocker spaniels with dilated cardiomyopathy. Journal of Small Animal Practice 48, 258-263
- Monnet, E., Orton, C. E., Salman, M., et al. (1995) Idiopathic dilated cardiomyopathy in dogs: survival and prognostic indicators. Journal of Veterinary Internal Medicine 9, 12-17
- O’Sullivan, M. L., O’Grady, M. R., Pyle, W. G., et al. (2011) Evaluation of 10 genes encoding cardiac proteins in Doberman pinschers with dilated cardiomyopathy. American Journal of Veterinary Research 72, 932-939
- Owczarek-Lipska, M., Mausberg, T. B., Stephenson, H., et al. (2013) A 16-bp deletion in the canine PDK4 gene is not associated with dilated cardiomyopathy in a European cohort of Doberman pinschers. Animal Genetics 44, 239
- Oxford, E. M., Everitt, M., Coombs, W., et al. (2007) Molecular composition of the intercalated disc in a spontaneous canine animal model of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm 4, 1196-1205
- Oxford, E. M., Danko, C. G., Kornreich, B. G., et al. (2011) Ultrastructural changes in cardiac myocytes from boxer dogs with arrhythmogenic right ventricular cardiomyopathy. Journal of Veterinary Cardiology 13, 101-113
- Oxford, E. M., Danko, C. G., Fox, P. R., et al. (2014) Change in β-catenin localization suggests involvement of the canonical Wnt pathway in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. Journal of Veterinary Internal Medicine 28, 92-101
- Oyama, M. A., Reiken, S., Lehnart, S. E., et al. (2008) Arrhythmogenic right ventricular cardiomyopathy in boxer dogs is associated with calstabin2 deficiency. Journal of Veterinary Cardiology 10, 1-10
- Perego, M., Ramera, L. & Santilli, R. A. (2012) Isorhythmic atrioventricular dissociation in Labrador retrievers. Journal of Veterinary Internal Medicine 26, 320-325
- Philipp, U., Broschk, C., Vollmar, A., et al. (2007) Evaluation of tafazzin as candidate for dilated cardiomyopathy in Irish wolfhounds. Journal of Heredity 98, 506-509
- Philipp, U., Vollmar, A., Häggström, J., et al. (2012) Multiple loci are associated with dilated cardiomyopathy in irish wolfhounds. PLoS One 7, 1-6
- Pion, P., Sanderson, S. & Kittleson, M. (1998) The effectiveness of taurine and levocarnitine in dogs with heart disease. Veterinary Clinics of North America: Small Animal Practice 28, 1495-1514
- Posafalvi, A., Herkert, J. C., Sinke, R. J., et al. (2013) Clinical utility gene card for: dilated cardiomyopathy (CMD). European Journal of Human Genetics 21
- Sanderson, S. L. (2006) Taurine and carnitine in canine cardiomyopathy. Veterinary Clinics of North America: Small Animal Practice 36, 1325-1343
- Sargan, D. (2015) Inherited diseases in boxer dogs: a cautionary tale for molecular geneticists (Editorial). Veterinary Record 176, 490-491
- Simpson, S., Edwards, J., Emes, R. D., et al. (2015) A predictive model for canine dilated cardiomyopathy—a meta-analysis of Doberman pinscher data. PeerJ 3, e842
- Simpson, S., Dunning, M., Brownlie, S., et al. (2016) Multiple genetic associations with Irish wolfhound dilated cardiomyopathy. BioMed Research International 2016, 1-14
- Sisson, D. D. & Thomas, W. P. (1995) Myocardial disease. In: Textbook of Veterinary Internal Medicine. 4th edn. Eds S. J. Ettinger and E. C. Feldman. WB Saunders, Philadelphia, PA, USA. pp 995-1032
- Sosa, I., Estrada, A. H., Winter, B. D., et al. (2016) In vitro evaluation of mitochondrial dysfunction and treatment with adeno-associated virus vector in fibroblasts from Doberman pinschers with dilated cardiomyopathy and a pyruvate dehydrogenase kinase 4 mutation. American Journal of Veterinary Research 77, 156-161
- Stephenson, H. M., Fonfara, S., López-Alvarez, J., et al. (2012) Screening for dilated cardiomyopathy in Great Danes in the United Kingdom. Journal of Veterinary Internal Medicine 26, 1140-1147
- Steudemann, C., Bauersachs, S., Weber, K., et al. (2013) Detection and comparison of microRNA expression in the serum of Doberman pinschers with dilated cardiomyopathy and healthy controls. BMC Veterinary Research 9, 12
- Summerfield, N. J., Boswood, A., O’Grady, M. R., et al. (2012) Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman pinschers with preclinical dilated cardiomyopathy (the PROTECT study). Journal of Veterinary Internal Medicine 26, 1337-1349
- Swift, S., Baldin, A. & Cripps, P. (2017) Degenerative valvular disease in the Cavalier King Charles spaniel: results of the UK breed scheme 1991-2010. Journal of Veterinary Internal Medicine 31, 9-14
- Tarducci, A., Borgarelli, M., Zanatta, R., et al. (2003) Asymptomatic dilated cardiomyopathy in Great Danes: clinical, electrocardiographic, echocardiographic and echoDoppler features. Veterinary Research Communications 27 (Suppl 1), 799-802
- Thomas, R. E. (1987) Congestive cardiac failure in young Cocker Spaniels (a form of cardiomyopathy?): details of eight cases. Journal of Small Animal Practice 28, 265-279
- Tidholm, A. & Jönsson, L. (1996) Dilated cardiomyopathy in the Newfoundland: a study of 37 cases (1983-1994). Journal of the American Animal Hospital Association 32, 465-470
- Tidholm, A. & Jönsson, L. (1997) A retrospective study of canine dilated cardiomyopathy (189 cases). Journal of the American Animal Hospital Association 33, 544-550
- Tidholm, A. & Jönsson, L. (2005) Histologic characterization of canine dilated cardiomyopathy (Review Article). Veterinary Pathology 42, 1-8
- Uchida, S. & Dimmeler, S. (2015) Long noncoding RNAs in cardiovascular diseases. Circulation Research 116, 737-750
- Vollmar, A. C. (2000) The prevalence of cardiomyopathy in the Irish wolfhound: a clinical study of 500 dogs. Journal of the American Animal Hospital Association 36, 125-132
- Vollmar, A. C. & Fox, P. R. (2016) Long-term outcome of Irish wolfhound dogs with preclinical cardiomyopathy, atrial fibrillation, or both treated with pimobendan, benazepril hydrochloride, or methyldigoxin monotherapy. Journal of Veterinary Internal Medicine 30, 553-559
- Werner, P., Raducha, M. G., Prociuk, U., et al. (2008) A novel locus for dilated cardiomyopathy maps to canine chromosome 8. Genomics 91, 517-521
- Wess, G., Schulze, A., Butz, J., et al. (2010) Prevalence of dilated cardiomyopathy in Doberman pinschers in various age groups. Journal of Veterinary Internal Medicine 24, 533-538
- Wess, G., Domenech, O., Dukes-McEwan, J., et al. (2017) European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman pinschers. Journal of Veterinary Cardiology 19, 405-415
- Wiersma, A. C., Leegwater, P. A., van Oost, B. A., et al. (2007) Canine candidate genes for dilated cardiomyopathy: annotation of and polymorphic markers for 14 genes. BMC Veterinary Research 19, 28
- Wiersma, A. C., Stabej, P., Leegwater, P. A. J., et al. (2008) Evaluation of 15 candidate genes for dilated cardiomyopathy in the Newfoundland dog. Journal of Heredity 99, 73-80
- Wotton, P. R. (1999) Dilated cardiomyopathy (DCM) in closely related boxer dogs and its possibly resemblance to arrhythmogenic right ventricular cardiomyopathy (ARVC) in humans. Proceedings of the 17th Annual Veterinary Medical Forum. ACVIM, Chicago, IL, USA. pp 88-89.
^Наверх









