Investigation of ventricular pre-excitation electrocardiographic pattern in two horses: clinicalpresentation and potential causes

Author information
Viu J., Armengou L., Decloedt A., Jose-Cunilleras E. Investigation of ventricular pre-excitation electrocardiographic pattern in two horses: clinicalpresentation and potential causes // J Vet Cardiol. 2018 Jun;20(3):213-221.
Abstract
Two horses referred to the Unitat Equina, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, for unrelated clinicalproblems, and with no previous history of cardiac disease exhibited an intermittent ventricular pre-excitation electrocardiographic patternduring hospitalization. Both animals showed decreased plasma total and ionized magnesium concentrations, but no other relevant electrolyte disturbances were detected. Altered interventricular septal motion associated with ventricular pre-excitation beats (VPBs) was detected on M-mode echocardiography in both horses. The likely localization of an accessory pathway (AP) was identified in case 2 using pulsed-wave tissue Doppler imaging in the left anterior paraseptal location. Decreased frequency of the VPB was observed with long-term magnesium supplementation and restoration of plasma magnesium concentrations. The presence of ventricular pre-excitation electrocardiographicpattern was attributed to higher sensitivity of the AP to hypomagnesemia in both cases.
KEYWORDS: Arrhythmia; Equine; Magnesium; Wolff–Parkinson–White syndrome
List of abbreviations
- AP accessory pathway
- ECG electrocardiogram
- iMg ionized magnesium
- PW-TDI pulsed-wave tissue Doppler image
- RP refractory period
- TDI tissue Doppler image
- TMg total magnesium
- VPB ventricular pre-excitation beats
- WPW Wolff—Parkinson—White syndrome
Case 1
A 5-year-old french saddle gelding was referred to Unitat Equina, Fundacio Hospital Clinic Veterinari, Universitat Autonoma de Barcelona, for surgical colic. During the post-operative period, the horse had intermittent fever and, on day 11, suddenly collapsed in the stall. At that point, the horse was presented with tachycardia (58 bpm), tachypnea (28 bpm), low PCV (16%), and decreased total plasma magnesium concentration (TMg = 0.59 mmol/L, reference value 0.7—1 mmol/L) [1], with normal plasma ionized calcium concentration. On resting electrocardiogram (ECG), abnormal morphology of QRS complexes was detected with a short PR interval (Fig. 1A; Table 1) and occasional normal beats (sinus rhythm with ventricular pre-excitation beats).
Subsequently, the ECG was continuously monitored using a telemetric system.d
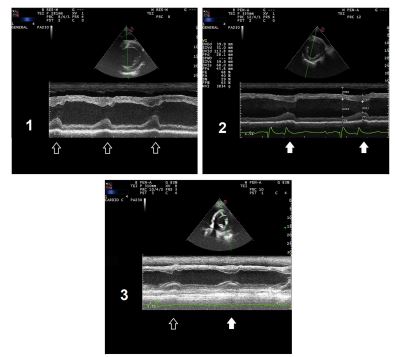
Intravenous magnesium sulfatee and oral magnesium sulfate heptahydrate (0.2 g/kg q 8 h), oral propranolol f (0.5 mg/kg q 8 h) and intravenous lidocaine g infusion (bolus of 1.3 mg/kg followed by 0.05 mg/kg/h) were administered to normalize plasma magnesium concentration and cardiac conduction. During treatment, normal beats were occasionally identified on the ECG, but sustained mild sinus tachycardia remained (Fig. 1B). On M- mode echocardiography,h abnormal septal move¬ment was identified during mechanical systole when the ventricular pre-excitation pattern was present (Fig. 2A).
dTelevet®100 - Telemetric ECG & Holter, Kruuse, Marslev, Germany
e Sulfate magnesium Lavoisier® 15%, Paris, France f Sumial® 40 mg AstraZeneca, Madrid, Spain g Laocaine® 20 mg/ml Intervet Schering-Plough animal health (MSD Animal Health, Kenilworth, NJ, USA.)
Plasma cardiac troponin I concentration was normal (<0.03 ng/mL, reference values <0.2 ng/mL) [2]. With this echocardio- graphic information and after observing persistent short PR intervals during a 24-h continuous ECG recording, a definitive electrocardiographic diagnosis of intermittent ventricular pre-excitation pattern was reached. The clinical condition of the horse improved, but at discharge after 20 days, the animal maintained mild sinus tachycardia (48 beats per minute) with all QRS complexes conducted through the accessory pathway (AP), and still had low plasma TMg* i concentration (0.6 mmol/L) and decreased urinary magnesium fractional excretion (9.82%, reference interval 15—35%) [3]. The horse was treated at the livery yard with oral magnesium sulfate heptahydrate (0.2 g/kg/day).
One month after being discharged and under oral magnesium supplementation, the horse was re-examined and had normal plasma TMg concentration and normal magnesium urinary fractional excretion. The heart rate was lower (36 bpm), and no alterations were detected on ECG recording (Table 1) or echocardiography at rest (Fig. 2B).
Case 2
A 6-year-old endurance Arabian mare was admit¬ted to Unitat Equina, Fundacio Hospital Clinic Veterinari, Universitat Autonoma de Barcelona, due to a lacerating wound of the left hind limb involving the digital flexor tendons. On cardiac auscultation, no abnormalities were audible other than a louder first heart sound. An ECG obtained before surgery revealed alternation between normal and ventricular pre-excitation beats (VPB;
h Mylab 70 Xvision® Esaote Espana, Barcelona, Spain
i Catlyst, Idexx laboratories, Inc, Netherlands
Table 1 Measures of normal and ventricular pre-excitation beats (VPBs) at rest and during exercise.
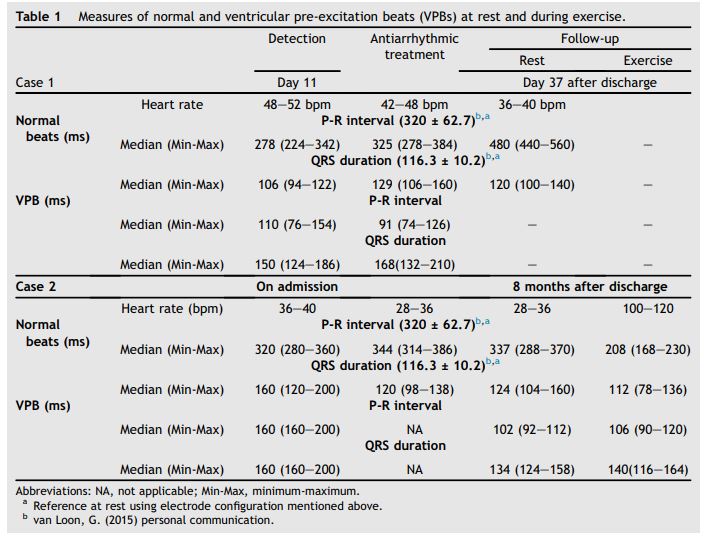
Fig. 1C). No alterations were found on routine plasma biochemistry or electrolyte concentrations (sodium, potassium, and ionized calcium). A decrease in plasma TMg concentration (0.34 mmol/L) was observed
The alternation of normal and VPB was present occasionally during anesthesia while receiving lidocaine as a con¬tinuous rate infusion, and during the immediate post-operative period without antiarrhythmic treatment. Post-surgery plasma cardiac troponin I concentration was normal (0.09 ng/mL; reference value < 0.2 ng/mL), and M-mode echocardiography showed abnormal septal movement during VPB (Fig. 2C).
Thirty-six hours after surgery, plasma TMg concentration and ECG were normal (Fig. 1). The mare improved progressively, and 8 months after surgery was back in endurance training without musculoskeletal sequelae. At that time, telemetric ECG evaluation was performed during exercise. Plasma-ionized calcium and magnesium concentrations were assessed before and immediately after a routine training session lasting 60 min in duration over a distance of 12 km, which included 30 min of walking, 15 min of trotting, and 15 min of canter. Abnormal heartbeats with ven¬tricular pre-excitation were detected during exercise and the recovery period (Fig. 1). Low plasma-ionized magnesium (iMg)j concentration was detected only after training (0.41 mmol/L after training; reference value > 0.47 mmol/L). Resting iMg was normal (0.49 mmol/L). Total magnesium determination was also performed resulting in a similar interpretation (normal values before exercise- 0.82 mmol/L and decreased after training 0.66 mmol/L) [1]. Owing to these findings, oral supplementation of magnesium sulfate heptahydrate was initiated (0.06 mg/kg/day). Electro¬cardiographic recording and plasma iMg concentration were periodically assessed during and after exercise (training and competition), and oral supplementation of magnesium sulfate hepta- hydrate was adjusted up to 0.18 g/kg/day. The mare did not show exercise intolerance at any time but continued to have VPB while exercising and during the recovery period. Once normal plasma iMg concentration was reestablished (0.48 mmol/L), the mare showed a normal ECG recording at rest and in a quiet environment. However, when exposed to an excitatory stimulus (i.e. clapping hands and yelling in front of the mare), VPB were detected at rest. The stimulus was repeated multiple times with consistent re-appearance of VPB every time. Once the animal calmed down, VPB disappeared.
j Nova 8 Analyzer ® Nova Biomedical Corporation 200 Prospect Street, Waltham MA, 02454-9141. USA
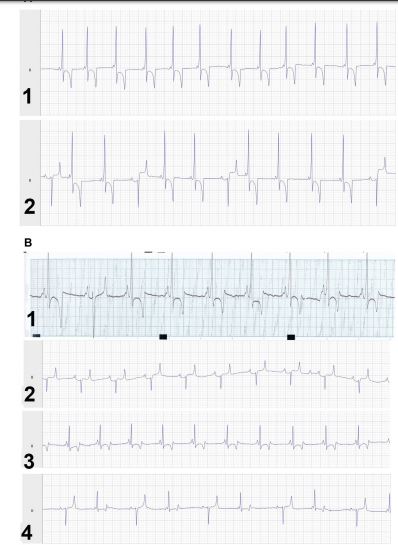
Figure 1 A: All electrocardiograms (ECGs) shown are recorded with lead II and 10 mV gain
1) Case 1: ECG on detection of ventricular pre-excitation beat (VPB; day 11 of hospitalization), mild supraventricular tachycardia, ECG is presented at 25 mm/s paper speed, heart rate 44 bpm; 2) Case 1: ECG during antiarrhythmic treatment (day 13), mild sinus tachycardia with normal and VPB, ECG is presented at 25 mm/s paper speed, heart rate 48bpm; B: 1) Case 2: ECG on detection of ventricular pre-excitation ECG pattern (on presentation to the hospital), supraventricular tachycardia with normal and VPB, ECG is presented at 50 mm/s paper speed, Heart rate 86 bpm; 2) Case 2: ECG after 36 hours of admission with normal TMg concentration, normal sinusal rhythm, ECG is presented at 25 mm/s paper speed, Heart rate 40 bpm; 3) Case 2: ECG during low-intensity exercise, supraventricular rhythm with normal heart rate (40 bpm). ECG is presented at 25 mm/s paper speed; 4) Case 2: ECG during recovery period (after endurance training for 12 km), supraventricular rhythm with normal heart (36 bpm) rate and alternance of normal and VPB.ECG is presented at 25 mm/s paper speed.
Figure 2 1) Case 1: M-mode echocardiography during VPB. Note the alteration of early systolic septal movement; 2) Case 1: M-mode echocardiography after ECG normalization. 3) Case 2: M-mode echocardiography showing a VPB and a subsequent normal one. Note the difference present in septal movement during early systole between the different beats. Ventricular pre-excitation beats are marked with open arrow and normal beats are marked with a solid one . ECG, electrocardiogram; VPB, ventricular pre-excitation beat.
Continuous electrocardiographic examination and determination of plasma iMg concentration was performed at rest and during endurance exercise on three routine training sessions lasting 60—110 min in duration over a distance of 12—18 km, which included 30 min of walking, 15—60 min of trotting, and 15—20 min of canter. The frequency of VPB was not clearly related to distance or plasma iMg concentration. Mean heart rate during exercise was also recorded, and no recognizable pattern was detected between peri¬ods with and without VPB. The frequency of VPB at rest (heart rate 28—36 bpm) was highly variable (0%—50%), whereas during exercise recovery (56—120 bpm), the frequency of VPB was higher (61%—100%). After 6 months of magnesium supplementation, the VPB appeared only sporadically during the recovery period (<0.5%). The PR interval and QRS duration of VPB and normal beats are shown in Table 1.
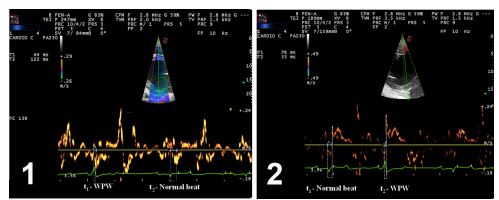
Twelve months after initial hospital admission an echocardiographic re-examination was per¬formed including the assessment of myocardial motion using pulsed-wave (PW) tissue Doppler imaging (TDI) to localize the AP. Using this technique, tissue velocity curves were recorded from different myocardial regions to identify the site of earliest mechanical activation (AP; Fig. 3). The study was performed with an ultrasound unit with a phased-array transducer at a frequency of 1.6/ 4 MHz. Pulsed-wave velocity measurements were performed on both right and left parasternal short- axis views of the left ventricle, interventricular septum, and right ventricle, at chordal level. The electromechanical time interval was measured as the time from the QRS complex to the time of systolic velocity peak. The shortest electro¬mechanical time interval detected on offline analysis was 33 ms (Fig. 3B) and corresponded to the right region of the left ventricular free wall, close to the interventricular septum or location 10 (left anterior paraseptal location) as described in human beings [5].
Figure 3 Pulse wave tissue doppler image evaluation of 1) right parasternal short-axis view at chordal level of the right and left ventricles, with the focus positioned in interventricular septal wall and 2) left parasternal short-axis view at chordal level of the left ventricle, with the focus positioned in the right region of the left ventricular wall, close to the interventricular septum. T1 and t2 depict time of electromechanical activation in different beats (normal and VPB, respectively). Note the shorter time (t2) of electromechanical activation on image 2. Dotted lines depict the interval between the beginning of electrical activation and the beginning of mechanical systole in each beat. VPB, ventricular pre-excitation beat.
Discussion
The presence of APs in the heart and the abnormal conduction of electric stimuli are uncommon con¬ditions in humans (0.1—0.3%) [6] and only a few cases have been reported in other species [7—13]. Intermittent preexcitation, or the alternation of normal sinus complexes with those exhibiting ventricular preexcitation, is the most common reported presentation of accessory pathways in horses [9,10,12,13].
This intermittent manifestation of antegrade accessory pathway conduction in humans is typically attributed to longer antegrade effective refractory periods (RPs) of the AP, resulting in pre-excitation manifested at slower sinus rates and disappearing at faster rates [14]. In addition, antegrade conduction and RP in some APs demonstrate marked sensitivity to autonomic tone [6] and can be influenced by electrolyte balance as well. Some authors suggest that the susceptibility of some APs to catecholamines is due to the presence of b-adrenergic receptors [15]. In the second case reported here, a clear relationship between autonomic nervous system tone and VPB was observed during a stress test using a repeated excitatory stimulus. During this test, plasma concentrations of electrolytes related to myocardial conduction, including iMg, were normal. The influence of emotional state on ventricular pre-excitation pattern has been discussed in a previous case report, in which a clear relationship could not be established [12]. Exercise-influenced autonomic tone was also a triggering factor for VPB in case two. Exercise-induced ventricular pre-excitation syndrome is reported in human beings [16,17] and seems to be related to hormones that increase during exercise (cortisol and catecholamines). In case 2, VPB appeared to be triggered by the effect of these substances, but the appearance of VPB was not related to a specific heart rate as is reported in human beings [6]. Senta et al. [10] also described a tendency to increase the frequency of VPB after exercise in a Thoroughbred horse, as in case 2, but monitoring during exercise was not performed. Unfortunately, no electrolyte deter¬minations were available during ventricular pre¬excitation ECG pattern in the cases described in the literature.
Plasma iMg concentration may also have an effect on the RP. It is especially important in some human patients with Wolff—Parkinson—White (WPW) syndrome, in which an increase in iMg or an increase in plasma potassium concentration may block conduction through the AP [18]. Ionized magnesium is a critical cofactor of the sodium/ potassium-ATPase pump and also regulates specific potassium channels. Decreased plasma iMg concentration produces a decrease in the activity of sodium/potassium-ATPase pump and the opening of inward rectifying potassium channels. As a consequence, repolarization time is prolonged, which leads to increased RP [4]. Other possible causes of lengthened action potential duration due to low iMg could be surface charge effect or more probably unblocking of calcium channels [19].
In human WPW, the sensitivity of atrioventricular node and accessory pathways to electrolyte imbalances can be different, leading to changes in preferential conduction. The first case in this report presented with hypomagnesemia during VPB, with no other electrolyte derangements. The normalization of the ECG (or marked decrease in VPB) after long-term oral magnesium supplementation suggests a possible effect of magnesium concentration on ventricular pre-excitation ECG pattern occurrence in this case. However, ECG morphology was checked with a standard ECG recording and not continuous 24-h telemetric ECG. Given that intermittent ventricular pre-excitation can exhibit day-to-day changes in the frequency of VPB, a standard ECG recording could be misleading.
The PR interval of the VPB observed in both cases, approximately 100 ms, was shorter than the PR interval described in two reports (approx¬imately 180 ms [12] or 230 ms [10]) but similar to that described in others (130 ms) [9]. In case 1, the treatment with antiarrhythmic drugs increased the duration and variability of the PR interval in normal sinus beats, but no effect was observed in VPB. This poor response of VPB to certain antiar¬rhythmic drugs has been previously reported in human beings [6] and horses [9].
In case 2, pulsed-wave tissue Doppler imaging (PW-TDI) was used to localize the AP within the right region of the left ventricular free wall, close to the interventricular septum. Using PW-TDI, a myocardial region with early activation during VPB could be identified, which was considered likely to be the region of the AP. However, in humans, localization of the AP in WPW is confirmed with intracardiac electrocardiographic mapping and PW-TDI is not considered the gold standard method [20]. Clinically, the most relevant concern for human patients with WPW is the development of a rapid, narrow complex tachycardia using the accessory pathway as the retrograde limb (most commonly) known as orthodromic atrioventricular reciprocating tachycardia, a rapid wide complex tachyarrhythmia known as antidromic reciprocating tachycardia (uncommon), or development of atrial fibrillation that conducts down the accessory pathway rapidly resulting in ventricular fibrillation.
Congestive heart failure is also described in horses due to sustained tachycardia (over 100 bpm) [21], but all horses with ventricular pre-excitation ECG pattern described in the literature did not maintain increased heart rates during long periods and did not develop cardiac insufficiency. In cases of ventricular pre-excitation syndrome in humans in which ablation of the AP is indicated, before surgery, the location of the AP is generally confirmed by intracardiac electro¬cardiographic mapping (gold standard). Unfortunately, this technique is not currently available for horses.
Wolff—Parkinson—White syndrome is described in human athletes and does not result in decreased performance unless tachyarrhythmias are present during exercise [16]. In the equine literature, three of the evaluated horses with ventricular pre-excitation ECG pattern were athletes evaluated before and after exercise. No ECG recording during exercise was available, therefore the presence of arrhythmias during exercise could not be ruled out. One of the previously reported cases was presented for investigation of poor performance [9] but in the other 2 cases, ventricular pre-excitation ECG pattern was an incidental finding. These animals did not have problems during competition [12] even when followed for a few years [10]. In case 2 of our report, athletic performance was unaffected by the VPS, and it is the first equine case of ventricular preexcitation ECG pattern in which exercising ECG was performed. In human beings with WPW, an increased tendency to present with atrial fibrillation was observed [22]. Although atrial fibrillation occurs commonly in horses and has been reported in horses with ventricular pre-excitation ECG pattern [23], this mare did not show any associated arrhythmia during training or competition. The previously reported cases showed an increased frequency of abnormally conduced beats after exercise [10,12], as was detected in case 2 of our report. The cause of this increase is not clear, and electrolyte concentrations were not available to assess their influence on ventricular pre-excitation ECG pattern in previous reports.
Possible causes of this increase in the frequency of VPB could be electrolyte losses in sweat (especially magnesium), an increase of circulating catecholamines, or the combination of both factors predisposing the appearance of VPB. Using PW-TDI, a myocardial region with early activation during VPB could be identified, which is likely to be the region of the AP. Antiarrhythmic drugs were ineffective in normalizing cardiac conduction. Administration of antiarrhythmic agents is also often not effective in humans with WPW, and the treatment of choice in severe cases is intracardiac electrical ablation of the AP [20]. Long-term magnesium supplementation and restoration of plasma magnesium concentration was associated with a decrease in the frequency of VPB in the cases reported here. Our findings suggest that magnesium supplementation in cases of ventricular pre-excitation ECG pattern in horses with low iMg plasma concentrations might reduce the incidence of ventricular pre-excitation ECG pattern, and measurement of plasma magnesium concentration should be considered in cases of ventricular pre-excitation in horses.
Conflicts of Interest Statement
The authors do not have any conflicts of interest to disclose.
References
- Johansson AM, Gardner SY, Jones SL, Fuquay LR, Reagan VH, Levine JF. Hypomagnesemia in hospitalized horses. J Vet Intern Med 2003;17:860-7.
- Nath L, Anderson G. Serum cardiac troponin I concentrations in horses with cardiac disease. Aust Vet J 2012;90: 351-7.
- Stewart A. Magnesium disorders in horses. Vet Clin N Am Equine Pract 2011;27:149-63.
- Topf J, Murray P. Hypomagnesemia and hypermagnesemia. Rev EndocrMetab Disord 2003;4:195-206.
- Arruda MS, McClelland JH, Wang X, Beckman KJ, Widman LE, Gonzalez MD, Nakagawa H, Lazzara R, Jackman WM. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electro¬physiol 1998;9:2-12.
- Wakita R, Takahashi M, Ohe C. Occurrence of intermittent Wolff-Parkinson-White syndrome during intravenous seda¬tion. J Clin Anesth 2008;20:146-9.
- Hill B, Tilley L. Ventricular preexcitation in seven dogs and nine cats. J Am Vet Med Assoc 1985;187:1026-31.
- Roland RM, Estrada AH. ECG of the month. Ventricular preexcitation. J Am Vet Med Assoc 2006;228:1500-2.
- Muir W, McGuirk S. Ventricular preexcitation in two horses. J Am Vet Med Assoc 1983;183:573-6.
- Senta T, Amada A. Japan Racing Association Racehorse Health Research Institute Report. Wolff Parkinson White (ventricular pre-excitation syndrome) in a thoroughbred, vol. 4; 1967. p. 129-36.
- Endo Y, Tajima M. The Wolff-Parkinson-White syndrome in a Holstein-friesian cow. Nihon Juigaku Zasshi 1990;52: 1155-61.
- Cooper S. Ventricular pre-excitation (Wolff-Parkinson- White syndrome) in a horse. Vet Rec 1962;74:527-30.
- Delahanty D, Glaxier D. The Wolff-Parkinson-White (atrioventricular conduction) Syndrome in a horse. Ir Vet J 1959;5:17b-22b.
- Josephson M. Preexcitation syndromes. In: Josephson M, editor. Clinical Cardiac Electrophysiology: Techniques and Interpretations. Philadelphia: Lippincott Williams and Wilkins; 2008. p. 339-446.
- Horio Y, Matsuyama K. Blocking effect of verapamil on conduction over a catecholamine-sensitive bypass tract in exercise-induced Wolff-Parkinson-White syndrome. J Am Coll Cardiol 1984;4:186-91.
- Pelliccia A, Fagard R, Bjornstad HH, Anastassakis A, Arbustini E, Assanelli D, Biffi A, Borjesson M, Carre F, Corrado D, Delise P, Dorwarth U, Hirth A, Heidbuchel H, Hoffmann E, Mellwig KP, Panhuyzen-Goedkoop N, Pisani A, Solberg EE, van-Buuren F, Vanhees L, Blomstrom- Lundqvist C, Deligiannis A, Dugmore D, Glikson M, Hoff PI, Hoffmann A, Hoffmann E, Horstkotte D, Nordrehaug JE, Oudhof J, McKenna WJ, Penco M, Priori S, Reybrouck T, Senden J, Spataro A, Thiene G. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the study group of sports cardiology of the working group of cardiac rehabilitation and exercise physiology and the working group of myocardial and pericardial diseases of the European society of cardiology. Eur Heart J 2005;26:1422-45.
- Mezzani A, Giovannini T, Michelucci A. Effects of training on the electrophysiologic properties of atrium and accessory pathway in athletes with Wolff-Parkinson-White syndrome. Cardiology 1990;77:295-302.
- Sideris A, Galiatsu E. Effects of magnesium and potassium on Wolff-Parkinson-White syndrome. J Electrocardiol 1996; 29:11-5.
- Davidenko JM, Cohen L, Goodrow R, Antzelevitch C. Qui- nidine-induced action potential prolongation, early after depolarizations, and triggered activity in canine purkinje fibers effects of stimulation rate, potassium, and magne¬sium. Circulation 1989;79:674-86.
- Zipes DP, Dimarco JP, Gillette PC, Jackman WM, Myerburg RJ, Rahimtoola SH, Ritchie JL, Cheitlin MD, Garson A, Gibbons RJ, Lewis RP. Guidelines for clinical intracardiac electrophysiological and catheter ablation procedures: a report of the american college of cardiol- ogy/american heart association task force on practice guidelines (committee on clinical intracardiac electrophysiologic and catheter ablation procedures), developed in collaboration with the North american society of pacing and Electrophysiology. J Am Coll Cardiol 1995;26:555-73.
- Bonagura JD. Congestive heart failure in the horse. In: Proc. 11th Am Coll Vet Intern Med Forum; 1993. p. 614-8.
- Cai Q, Shuraih M, Nagueh S. The use of echocardiography in Wolff-Parkinson-White syndrome. Int J Cardiovasc Imag 2012;28:725-34.
- Jesty S, Kraus M, Johnson A. An accessory bypass tract masked by the presence of atrial fibrillation in a horse. J Vet Cardiol 2011;13:79-83.
^Наверх









