Extracardiac intrapericardial myxosarcoma causing right ventricular outflow tract obstruction in a dog

Author information
Karlin E.T., Yang V.K., Prabhakar M., Gregorich S.L., Hahn S., Rush J.E. Extracardiac intrapericardial myxosarcoma causing right ventricular outflow tract obstruction in a dog . J Vet Cardiol. 2018 Apr;20(2):129-135.
Abstract
A 13-year-old male castrated pomeranian cross was referred for evaluation of episodes of collapse and a suspected cardiac mass. The presence of a mass at the base of the heart within the pericardial space was confirmed by echocardiography. Additional diagnostics included computed tomography, ultrasound-guided fine-needle aspirate, and thoracic radiographs. The mass was surgically debulked and diagnosed as myxosarcoma via histopathology. This case report describes the diagnostic imaging, laboratory findings, and short-term positive clinical outcome of a dog with a myxosarcoma in a previously undescribed location.
KEYWORDS: Cardiac mass; Cardiac neoplasia; Heart base tumor; Right-sided congestive heart failure
Abbreviations
- CHF congestive heart failure
- CT computed tomography
- PA pulmonary artery
- RV right ventricle
- RVOT right ventricular outflow tract
A 13-year-old, 3-kg, male castrated pomeranian cross presented to the Foster Hospital for Small Animals at Tufts University Cummings School of Veterinary Medicine for episodes of collapse and increased coughing. He also had a history of cough that was thought to be related to tracheal collapse. The cough was not historically responsive to antimicrobial therapy but improved with prednisone and cough suppressant medication.
On presentation, the dog was bright and alert with normal vital parameters. Physical examination revealed mild tachycardia (160 beats per minute), mild dyspnea (40 breaths per minute with mildly increased respiratory effort), a IN—IV/VI systolic heart murmur with the point of maximum intensity over the right and left apical region, and a mid¬systolic click. There was jugular venous distension. Serum biochemistry profile and complete blood count had been performed by the referring veterinarian, and the results were within reference intervals except for blood urea nitrogen of 31 mg/ dL (reference interval 7—27 mg/dL), alanine aminotransferase of 139 U/L (reference interval 10—125 U/L), cholesterol of 106 mg/dL (reference interval 110—320 mg/dL), hematocrit of 36.4% (reference interval 37.3—61.7%), and lactate of 4.3 mmol/L (reference interval 0.3—2.0 mmol/L). The patient was admitted for diagnostics including echocardiogram, computed tomography (CT) scan, thoracocentesis, and fine-needle aspirate.
The echocardiograma was performed while the dog was standing due to excessive coughing when the dog was restrained in lateral recumbency. A large, lobulated, hypoechoic mass (approximately 3 cm x 4.5 cm) was visualized adjacent to the right heart, causing dramatic compression of the right ventricle (RV) and pulmonary artery (PA; Fig. 1A, Video 1). The mass was within the pericardium and located near the heart base, but the origin of the mass could not be determined via echocardiography (Fig. 1B). There was hyperdynamic contractile function (fractional shortening 56%) of the left ventricle, and the left atrium was mildly dilated. The RV free wall was subjectively thickened, and RV papillary muscles were prominent. There was thickening of the mitral and tricuspid valves with moderate mitral and tricuspid regurgitation. The regurgitant velocity across the tricuspid valve was elevated at 5.2 m/s (reference interval < 2.5 m/ sec), corresponding to an estimated RV to right atrium pressure gradient of 107 mmHg [1]. The maximum right ventricular outflow tract (RVOT) velocity adjacent to the mass was elevated at 3.4 m/s (pulmonic outflow velocity reference interval 1.05 ± 0.19 m/s) [1].
These values were suggestive of moderate pulmonary hypertension (estimated PA pressure of 70—75 mmHg, based on an estimated right atrial pressure of 10—15 mmHg), consistent with a history of chronic respiratory dis¬ease. There was moderate pleural effusion, small volume pericardial effusion, and scant ascites. The effusions were presumed to be indicative of right¬sided congestive heart failure (CHF) secondary to obstruction of blood flow across the RVOT caused by compression of the right heart by the mass (Video 2). Based on echocardiography, differential diagnoses for the mass included ectopic thyroid carcinoma, sarcoma, chemodectoma, and hemangiosarcoma. However, the echocardiographic appearance of the mass was considered atypical of these more com¬monly seen cardiac tumors.
Computed tomography examination was performed with a 16-slice CT scanner.b Volume data of the thorax were obtained before and after the administration of 2 mL/kg Iohexol (300 mg I/mL) IV. Reconstructed images (slice thickness and index = 2 mm) were evaluated using lung and soft tissue algorithms in transverse, sagittal, and dorsal planes. Images showed an approximately 4.1 cm x 3.2 cm x 5.7 cm mixed attenuation mass within the left aspect of the pericardium (Fig. 2A and B). Centrally, the mass was hypodense and contained ill-defined areas of patchy contrast enhancement. Peripherally and most notably along the ventral aspect, the mass was more hyperdense and minimally contrast enhancing. The mass was compressing the ventral aspect of the RV, the main PA, and the left caudal mainstem bronchus, caus¬ing rightward deviation of cardiac silhouette. Cardiac invasion was not seen. There was moderate thickening and contrast enhancement of the pericardium surrounding the mass. A moderate volume of bilateral pleural effusion was present. In addition, there was peritoneal effusion and caudal vena cava distension consistent with right-sided CHF. Unrelated to the mass, there was evidence of tracheal and mainstem bronchial collapse. Pul¬monary metastatic disease was not observed. Dif¬ferential diagnoses for the mass included round cell neoplasia, teratoma, sarcoma, pericardial branchial cyst, and mesothelioma [2].
a Vivid E9 GE Medical Systems, Milwaukee, WI, USA.
b Toshiba Aquilon 16, Toshiba American Medical Systems Inc., Tustin, CA, USA.
Figure 1 Echocardiographic images. (A) Right parasternal short axis view at the level of the heart base, angled to highlight the mass surrounding the base of the pulmonary artery
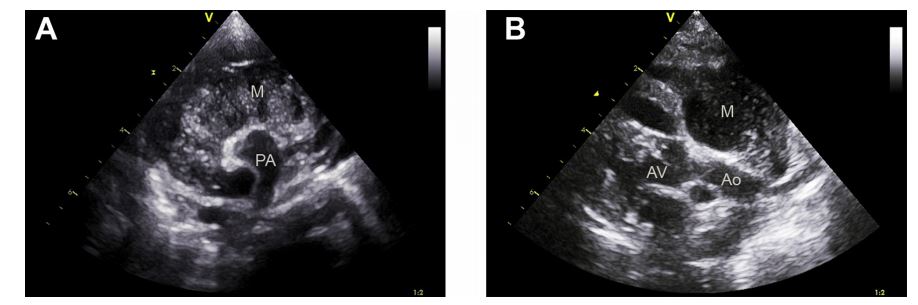
The hypoechoic, textured appearance of the mass is shown. (B) Right parasternal long axis view showing the mass located at the heart base in close proximity to the aorta. M, mass; PA, pulmonary artery; Ao, aorta; AV, aortic valve.
Figure 2 Transverse post contrast CT images (W: 320, L: 30) at the level of the left ventricle (A) and at the level of the ventricular outflow tract (B).
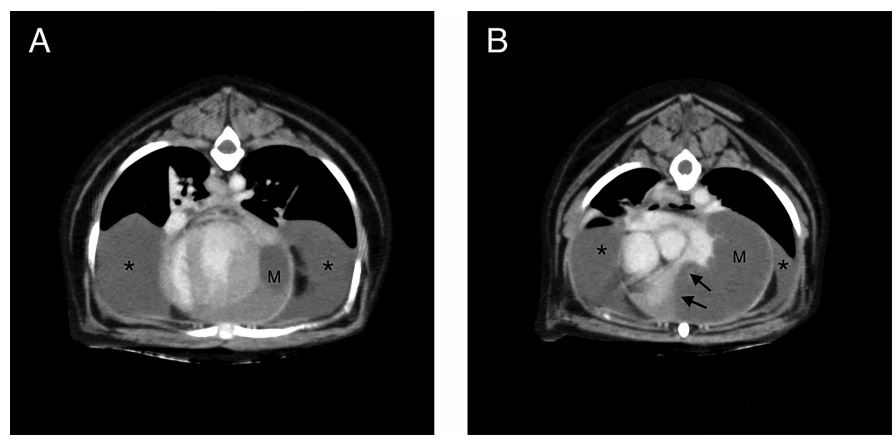
A mixed attenuation mass (M) can be seen within the left aspect of the pericardium. At the level of the right ventricular outflow tract, there is compression of the right ventricle and main pulmonary artery by the mass (arrows). The left aspect of the pericardium is thickened. A moderate amount of pleural effusion (*) is noted. Tracheal collapse is also noted in image A, unrelated to the mass. CT, computed tomography; M, mass.
Ultrasound-guided fine-needle aspirate of the mass was performed after the CT scan. Cytology was consistent with cystic fluid with chronic hemorrhage or blood contamination. Neoplastic cells were not seen. Thoracocentesis was performed, yielding 25 ml of serosanguinous fluid from the right hemithorax. Cytology was consistent with a modified transudate with hemorrhage or diapedesis consistent with right-sided CHF. However, due to the presence of large basophilic cells displaying morphology consistent with large mesothelial cells, mesothelioma or carcinoma were included in the differential diagnoses.
Urgent surgical exploration to relieve RVOT compression was recommended. The dog was discharged with medical therapy for right-sided CHF (furosemide 2 mg/kg PO q12h, pimobendan 0.2 mg/kg PO q12h, enalapril 0.8 mg/kg PO q24h) pending the owners’ decision to pursue surgery. The dog was presented 10 days later for pericardiectomy and possible mass removal or surgical debulking. The owners reported that the dog had been stable at home. Echocardiography revealed resolution of pleural effusion and ascites, and the mass was unchanged in appearance. A median sternotomy and subtotal pericardiectomy were performed.
An approximately 5 cm x 5 cm mass was visible at the base of the heart, within the pericardium. The tumor appeared locally invasive surrounding the great vessels.
Complete excision was judged not to be possible. A partial debulking excision of the mass was performed because of concern that clinical signs were a result of direct RVOT compression by the mass. It was estimated that at least 60% of the mass was removed by a combination of blunt dissection and electrocautery. The tumor had a gelatinous, sticky, partially firm consistency, and minimal hemorrhage was encountered during debulking (Fig. 3).
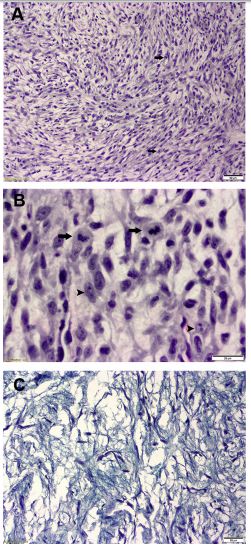
Microscopic examination was performed on formalin-fixed and paraffin-embedded tissues. The slides were routinely processed and stained with hematoxylin and eosin. A multilobular mass appeared to be emerging from a thick fibrous band, containing adipose tissue and lined by hyperplastic mesothelial cells. Cellular density varied significantly throughout the tumor. Most areas exhibited low cellularity and were composed of individualized spindle cells within abundant pale-gray amorphic stroma. Cellular areas were characterized by larger spindle-shaped or polygonal cells, exhibiting moderate pleomorphism, multiple nucleoli, and mitotic activity of 1—2 per high power field (Fig. 4A and B).
The slides were stained with Alcian blue, Alcian blue-PAS, and immunohistochemically for vimentin and S-100 protein. The stroma stained positive with Alcian blue, and weakly with PAS stain, supporting mucin of connective tissue origin (as opposed to mucin of epithelial origin, which would be positive for both stains; Fig. 4C). Vimentin is a marker for most mesenchymal cells, whereas S-100 protein is used to more specifically identify lipocytes, melanocytes, Schwann cells, and chondrocytes.
In this case, the neoplastic cells were positive for vimentin, but negative for S-100 protein, ruling out a chondrocytic or Schwann cell origin [3,4]. These findings were most consistent with a diagnosis of myxosarcoma [3—5].
Figure 3 Gross appearance of the multinodular gelatinous mass at the time of surgical debulking.
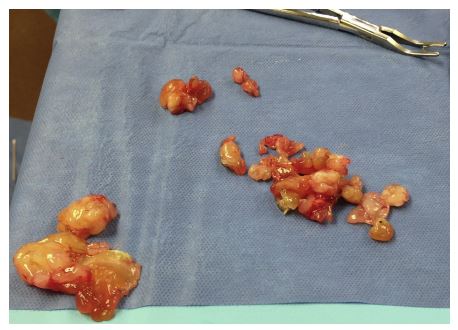
Figure 4 Biopsy samples. (A) Area of higher cellularity, showing cellular pleomorphism and mitotic figures (arrows); H&E, X10. (B) Two mitotic figures (arrows) and multiple nucleoli of the neoplastic cells (arrow heads); H&E, X20. (C) Alcian blue-PAS stain showing the blue stroma (positive for Alcian blue, negative for PAS), with nuclei of the neoplastic cells stained purple; Alcian blue- PAS, X10. H&E, hematoxylin and eosin stain; PAS, Periodic acid—Schiff stain.
One day after surgery, an echocardiogram did not reveal pleural effusion or ascites. The unresected portion of the mass was not clearly visible, and the RVOT was no longer compressed (video 3). The maximum RVOT velocity was normal at 1.2 m/ s. The tricuspid regurgitation velocity was not obtained due to difficulty in obtaining the necessary view without impinging on the patient’s incision and bandage. Furosemide and enalapril were discontinued. Two days after surgery, the patient was discharged with tramadol (4 mg/kg PO q8-12h) and pimobendan (0.2 mg/kg PO q12h).
A 2-week postoperative follow-up appointment with oncology was postponed by the owners. The dog presented to the oncology service for reevaluation 10.5 weeks after surgery. He had experienced two collapsing episodes since surgery and had required hydrocodone regularly to prevent cough but had otherwise been doing well with normal appetite and energy levels. Recheck echocardiogram revealed local tumor progression, measuring approximately 2.5 cm x 5 cm, with compression of the RV and main PA as visualized before surgery. Tricuspid regurgitation velocity was not obtained due to patient temperament, but the maximum RVOT velocity was elevated at 2.4 m/s. Neither pleural effusion nor ascites was observed. Adjunct chemotherapy or radiation therapy was declined by the owners. The dog was discharged with instructions to continue pimobendan and hydrocodone. Seventeen weeks after surgical resection of the mass, the dog died at home. Details regarding his clinical condition were unavailable.
Discussion
Primary cardiac tumors are uncommon in the dog, with a reported incidence of 0.19%. Hemangio- sarcoma and chemodectoma are most commonly reported. Only 8% of tumors in a large retrospective study were classified as a connective tissue neoplasms [6]. Myxoid neoplasms are connective tissue tumors that are uncommon both in people and in dogs [3]. They are of fibroblast origin but are characterized by a matrix rich in glycosaminoglycan and are unable to produce mature collagen [5]. Benign (i.e., myxoma) and malignant (i.e., myxosarcoma) forms have been reported in dogs in the heart, thorax, mesentery, subcutis, fascia, and joint synovia [7].
Several cases of cardiac myxosarcoma have been reported in dogs, but most are intracavitary masses arising from either the atrial wall or one of the atrioventricular valves [8—13]. Myxosarcomas and other cardiac sarcomas in retrospective stud¬ies of humans were all reported to be intracavitary [14,15]. Intrapericardial myxosarcoma associated with the left ventricle and right atrium have been reported in dogs [16,17]. This is the first report of an extracardiac intrapericardial myxosarcoma causing RVOT obstruction and right-sided CHF in the absence of significant pericardial effusion.
In humans and dogs, the prognosis for myx¬osarcoma is guarded due to local recurrence and/ or metastasis [14,15,17]. Surgical excision or par¬tial resection is often required to alleviate clinical signs resulting from the space-occupying nature of these tumors. Adjunct chemotherapy and/or radiation therapy may be utilized, but neither has been convincingly shown to have a survival benefit in humans [14,15].
In dogs with cardiac masses causing pericardial effusion, pericardiectomy or pericardial window is often recommended to alleviate the symptoms associated with cardiac tamponade [18]. In the present case, RVOT obstruction was thought to be the result of direct compression by the tumor and would not be alleviated by pericardiectomy alone. Further, the echocardiographic appearance of the mass was considered atypical, leading to optimism that surgical debulking might have been feasible.
Computed tomography provided additional diagnostic information to better define the loca¬tion of the mass. It also helped rule out the pres¬ence of metastatic disease in the chest wall or lungs that has been reported in both people and dogs with cardiac myxosarcoma [8,14,15]. In this case, the origin of the mass remained unclear based on echocardiography, CT, and percutaneous sampling alone. Thus, surgical exploration was required to determine the source of the tumor.
Histopathology ultimately provided definitive diagnosis of the tumor. Myxosarcomas are characterized by stellate or spindle cells with nuclear pleomorphism and high mitotic index and arranged in an abundant myxoid stroma [5]. The presence of mucin intracellular matrix was confirmed by positive staining with Alcian blue, consistent with previous reports of myxosarcoma [9,10,16,17]. The origin of the myxosarcoma in this case was suspected to be either the pericardium or cardiac skeleton (including adventitia of the great vessels) based on its location. These two structures cannot be differentiated histologically as both possess similar features (adipose tissue, collagen, and elastic fibers).
Right-sided CHF was suspected to be secondary to severe RVOT compression, resulting in outflow obstruction based on elevated RVOT and tricuspid regurgitation velocities and the absence of significant pericardial effusion. This finding was in contrast to other reports of myxosarcoma where right-sided CHF was associated with tumors generating pericardial effusion and right atrial tamponade [8,9,13,17]. Stenting of the RVOT was considered but not pursued due to concerns for stent fracture given the severity of dynamic outflow tract obstruction.
Chemotherapy and/or radiation therapy were declined by the owner, but it is unclear whether either modality would have altered outcome. The patient remained free of right-sided CHF without diuretic therapy. However, 10.5 weeks after surgical debulking, the mass was visible on echo¬cardiography and was similar to the original size. This clinical course is consistent with previous reports of myxosarcoma in both humans and animals [14,15,17]. A retrospective study of primary cardiac sarcomas in people showed that tumor recurrence was the cause of death in 50% of cases [15]. Despite local tumor progression and some degree of RVOT obstruction, this dog survived 17 weeks after surgical debulking.
This case illustrates several previously unreported findings. It describes an unusual extracardiac intrapericardial location of a myxosarcoma, originating from either the great vessels or the pericardium near the base of the heart, and causing right-sided CHF due to RVOT compression. While myxosarcoma occurs more often as an intracavitary mass, it should be included as a differential for tumors located at the heart base. This is important because the less vas¬cular nature of myxosarcomas makes palliative sur¬gical debulking more feasible. This case also demonstrates that pericardiectomy and surgical debulking can result in improved quality of life and decreased dependency on cardiac medications for a period of time despite local tumor progression.
Conflicts of Interest Statement
All other authors declare no conflict of interest.
References
- Boon JA. Veterinary echocardiography. 2nd ed. West Sus¬sex, UK: Wiley-Blackwell; 2011.
- Restrepo CS, Vargas D, Ocazionez D, Martinez-Jimenez S, Cuellar SLB, Gutierrez FR. Primary pericardial tumors. Radiographics 2013;33:1613-30.
- Sommerey CC, Borgeat KA, Hetzel U, Archer J, Torrance AG. Intrathoracic myxosarcoma in a dog. J Comp Pathol 2012;147:199-203.
- Headley SA, Faria dos Reis AC, Bracarense AP. Cutaneous myxosarcoma with pulmonary metastases in a dog. J Comp Pathol 2011;145:31-4.
- Goldschmidt M, Hendrick M. Tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors in Domestic Animals 4th Edition. Ames: Iowa State Press; 2002. p. 45-117.
- Ware W, Hopper D. Cardiac tumors in dogs: 1982-1995. J Vet Intern Med 1999;13:95-103.
- Parslow A, Taylor D, Simpson D. Clinical, computed tomographic, magnetic resonance imaging, and histologic findings associated with myxomatous neoplasia of the temporomandibular joint in two dogs Arana. JAVMA 2016; 249:1301-7.
- Briggs OM, Kirberger RM, Goldberg NB. Right atrial myx¬osarcoma in a dog. J S Afr Vet Assoc 1997;68:144-6.
- Machida N, Hoshi K, Kobayashi M, Katsuda S, Yamane Y. Cardiac myxoma of the tricuspid valve in a dog. J Comp Pathol 2003;129:320-4.
- Adissu H, Hayes G, Wood G, Caswell J. Cardiac myxosarcoma with adrenal adenoma and pituitary hyperplasia resembling Carney complex in a dog. Vet Pathol 2010;47: 354-7.
- Hsieh B, Cohen M, Levitzke B, Arndt J. What is your diag¬nosis. J Am Vet Med Assoc 2013;201:1093-4.
- Beal MW, Mcguire LD, Langohr IM. Axillary artery tumor embolism secondary to mitral valve myxosarcoma in a dog. J Vet Emerg Crit Care 2014;24:751-8.
- Kocaturk M, Salci H, Bicer M, Akkoc A, Sag S, Yilmaz Z. Diagnostic and therapeutic approach to cardiac myx¬osarcoma in a dog. Turkish J Vet Anim Sci 2016;40:521-5.
- Liu S, Wang Z, Chen AQ, Zhou GH, Jiang ZB, Xiao MD. Cardiac myxoma and myxosarcoma: clinical experience and immunohistochemistry. Asian Cardiovasc Thorac Ann 2002;10:8-11.
- Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow¬up of 24 cases. Histopathology 1999;34:295-304.
- Riegel CM, Stockham SL, Patton KM, Thomas CL. What is your diagnosis? Muculent pleural effusion from a dog. Vet Clin Pathol 2008;37:353-6.
- Foale RD, White R a S, Harley R, Herrtage ME. Left ven¬tricular myxosarcoma in a dog. J Small Anim Pract 2003;44: 503-7.
- Ehrhart N, Ehrhart EJ, Willis J, Sisson D, Constable P, Greenfield C, Manfra-Maretta S, Hintermeister J. Analysis of factors affecting survival in dogs with aortic body tumors. Vet Surg 2002;31:44-8.
^Наверх









