Clinical and electrocardiographic presentations of transient trifascicular block in three cats
Author information
Oxford E.M., Giacomazzi F.B., Moïse N.S., Santilli R.A. Clinical and electrocardiographic presentations of transient trifascicular block in three cats // J Vet Cardiol. 2018 Jun;20(3):204-212.
Abstract
This report describes transient trifascicular block in three cats presented with lethargy and inappetence, and elevated cardiac troponin I concentrations. The electrocardiogram (ECG) of cat 1 showed a sinus rhythm with pronounced first-degree atrioventricular (AV) block, right bundle branch block, and left anterior fascicular block. The ECG of cat 2 showed truncular left bundle branch block alternating with left anterior fascicular block coupled with prolonged PR intervals, second-degree heart block, and paroxysmal third-degree AV block. The ECG of cat 3 showed first-degree AV block with concomitant right bundle branch block. The diagnosis of trifascicular block was made when paroxysmal third-degree AV block was documented. All cats recovered with medical management within weeks. Each cat resumed a sinus rhythm. Elevated cardiac troponin I concentrations suggested myocarditis that improved.
Abbreviations
- AV atrioventricular
- cTnI troponin I
- ECG electrocardiogram
- FS fractional shortening
- IVSd interventricular septum diastole
- LA/Ao left atrium/aortic
- LAFB left anterior fascicular block
- LBBB left bundle branch block
- LVFWd left ventricular free wall diastole
- LVIDd left ventricular internal dimension diastole
- RBBB right bundle branch block
Case 1
A 6-year-old male castrated domestic short hair cat (8.1 kg) was referred to Cornell University with a 1-day history of lethargy, inappetence, and dyspnea. Vital signs included a heart rate that ranged from 120 to 150 beats/min, respiratory rate >60 breaths/min, and rectal temperature of 37.4 °C. Muffled heart sounds and harsh lung sounds were auscultated.
Thoracic radiographs showed enlarged pulmo¬nary veins with a diffuse, severe interstitial pattern consistent with pulmonary edema. Mild pleural effusion was also evident. Initial electrocardiogram (ECG) revealed third-degree atrioventricular (AV) block with an idioventricular rhythm and AV dissociation.
The cat was administered 1 mg/kg furosemide and 0.2 mg/kg butorphanol intravenously and placed in an oxygen cage. During the subsequent 12 h, two additional 2 mg/kg boluses of furosemide were administered. The respiratory rate stabilized and the heart rate remained between 120 and 140 beats/min.
A CBC and serum biochemistry were normal and FIV/FeLV were negative. Cardiac troponin I (cTnI)a was calibrated according to the manufacturer instructions. Cardiac troponin I was elevated (9.0 ng/mL [normal: 0—0.09 ng/mL]). The cTnI assay was done with an analyzer not validated for use in cats because of the highly conserved sequence of troponin across species. Echocardiography revealed asymmetrical left ventricular hypertrophy (interventricular septum diastole [IVSd]: 5.7 mm; left ventricular free wall diastole [LVFWd]: 6.9 mm; normal [1] < 6 mm, with a normal left ventricular chamber dimension; left ventricular internal dimension diastole [LVIDd]: 15.2 mm; normal [1] < 21 mm). The left atrium was not enlarged (left atrium/aortic [LA:Ao]: 1.4; normal [1] < 1.6). Systolic function was also normal (fractional shortening (FS): 74%; normal [1] > 60%). Trivial mitral regurgitation in the absence of noticeable valvular degeneration was present. Right atrial and ventricular dimensions were subjectively considered normal.
a Abbott point of care, 400 College Rd E, Princeton, NJ 08540, United States.
From a 6-lead electro¬cardiogram (ECG), sinus bradycardia (heart rate 120 beats/min) and first-degree AV block (PR interval: 180 ms; normal [2] < 90 ms) were diagnosed. In addition, the QRS complex was prolonged (60 ms; normal [2] < 40 ms) because of large S waves (rS pattern) in leads II, III, and aVF, but with a left axis shift (-74°; normal: 0°—160°) suggesting a bifascicular block (combination of complete right bundle branch block [RBBB] and left anterior fascicular block (LAFB; Fig. 1A). Also, paroxysmal third-degree AV block with AV dissociation and a ventricular escape rhythm was present (Fig. 1B). A diagnosis of trifascicular block was made because of (1) RBBB, (2) LAFB, and (3) concomitant paroxysmal AV block.
The cat remained hospitalized for 2.5 days, and serial cTnI concentrations and continuous electrocardiogram (ECG) were monitored. After 24 h, the cat was eupneic, and radiographs confirmed subtotal resolution of pulmonary edema. The heart rate remained above 160 beats/min. Six-lead ECG revealed the pres¬ence of trifascicular block, and cTnI was 10.2 ng/ mL. A sinus rhythm (180 beats/min) conducted with first-degree AV block (PR interval: 140 ms) was identified. The QRS complex was prolonged (60 ms; normal [2] < 40 ms) because of large S waves (rS pattern) in leads I, II, and aVF with a left axis shift (-74°; normal: 0°—160°) suggesting bifascicular block (combination of complete RBBB and LAFB. In addition, alternating with this pattern was a bifascicular (truncular) left bundle branch block (LBBB) with a wide QRS complex (60 ms) and normal mean electrical axis (+86°) for (Fig. 2A).
The cTnI concentrations were measured every 12—24 h during hospitalization and initially remained elevated, despite the improvements in heart rate and rhythm [12 h post-presentation: 9.0 ng/mL; 24 h post-presentation: 10.2 ng/mL; 36 h post-presentation 9.75 ng/mL; 50 h post-presentation: 3.94 ng/mL].
The cat was discharged with furosemide (1 mg/ kg PO q 24 h) and benazepril (0.25 mg/kg PO q 24 h). A 2-week evaluation revealed improved cTnI concentration (0.78 ng/mL). Electrocardiogram revealed sinus rhythm (214 beats/min) with normal PR interval, narrow QRS complex (30 ms), and a left axis shift (-56°), indicating LAFB only (Fig. 2B). The cat remained eupneic with a resting respiratory rate of 16 breaths per minute 1 month later. At this time, the owners were instructed to monitor the resting respiratory rate closely and to discontinue all medications (LAFB remained on ECG, cTnI concentration 0.3 ng/mL). Further imaging was not performed at this time due to financial limitations. The cat was seen for an 8- month reevaluation, at which time, no clinical signs of cardiac disease were reported. Electrocardiogram revealed sinus rhythm (180 beats/min) with normal PR interval, narrow QRS complex (30 ms), and a left axis shift (-56°), indicating LAFB (Fig. 2C). The cTnI was measured at 0.15 ng/mL.
Case 2
An 11-year-old spayed female domestic shorthair cat (7.1 kg) was referred for a 2-day history of anorexia, left head-tilt, and vomiting. One year before the cat had an episode of pancreatitis and a history of asthma that was treated with pre¬dnisolone (1 mg/kg PO q 24 h) until 2 days before admission. Vital signs included a heart rate of 200 beats/min, respiratory rate of 28 breaths/min, and a temperature of 37.6 C. A grade II/VI systolic murmur was auscultated over the left cranial sternum. Neurological abnormalities included vestibular ataxia with left head-tilt and positional rotational nystagmus with the fast phase to the right. These signs were interpreted to indicate left-sided peripheral vestibular disease, with main differentials including infection (otitis interna) or polyps.
Thoracic radiographs identified a small, cavitated soft tissue opacity in the right caudal lung lobe and an atelectatic right middle lung lobe. Biochemistry panel was unremarkable. The cTnI concentration was elevated (7.55 ng/mL). Tests for infectious diseases (FIV/FeLV, fungal serology for Cryptococcus, Neospora indirect fluorescence antibody titer, Toxoplasmosis, and PCR for tick- borne diseases) were negative.
Echocardiography revealed asymmetric hypertrophic cardiomyopathy with regional involvement of the interventricular septum and normal left ventricular chamber dimensions (IVSd: 7.8 mm; LVFWd: 4.1 mm; LVIDd: 14.1 mm). The left atrium was enlarged (LA:Ao: 1.75). Fractional shortening was 66%.
Six-lead ECG revealed sinus tachycardia (230 beats/min), first-degree AV block (PR inter¬val: 160 ms) and bifascicular truncular LBBB with wide QRS complex (60 ms) and a normal mean electrical axis (+63°). Alternating post-divisional left bundle branch block with narrow QRS complex (30 ms) and normal mean electrical axis (+45°), and monofascicular LAFB with narrow QRS complex (30 ms) and left axis shift (-39°) were noted. One instance of second-degree AV block was noted (Fig. 3A). The diagnosis of trifascicular block was based on the presence of alternating LAFB, LBBB and second-degree AV block.
The cat remained hospitalized for 2.5 days and responded well to medical management [intravenous fluid therapy (40 mL/kg/day); famotidine (0.5 mg/kg IV q 12 h); maropitant (1 mg/kg SQ q 24 h); clindamycin (22 mg/kg IV q 12 h)] with no continued vomiting. Diazepam was administered once (0.1 mg/kg) as an appetite stimulant, after which time the cat began eating.
Figure 1 A 6-lead electrocardiogram at admission of case 1. A. Notice sinus bradycardia with a rate of 120 beats/min, conducted with a pronounced first- degree atrioventricular block (bracket, PR interval: 180 ms)
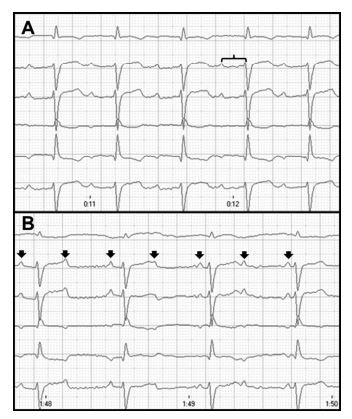
Wide QRS complex (60 ms) with left axis shift (-74°), suggesting the presence of a bifascicular block (complete right bundle branch block and left anterior fascicular block). B. Notice the presence of third-degree atrioventricular block with a ventricular escape rhythm of 100 beats/min and atrioventricular dissociation (black arrows marking P waves). At this time, cardiac troponin I was 9.0 ng/mL (ref 0—0.09 ng/mL). Speed 50 mm/s and calibration 10 mm/mV.
Figure 2 A. A 6-lead electrocardiogram 24 h after admission of case 1. Notice the presence of sinus rhythm with a rate of 180 beats/min conducted with first-degree atrioventricular block (bracket, PR interval: 140 ms).
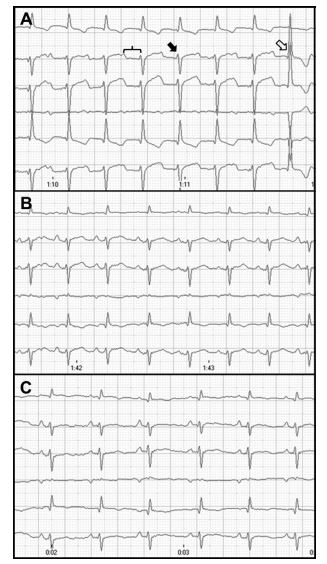
Alternating bifascicular block (combination of right bundle branch block [RBBB] and left anterior fascicular block [LAFB]) with large S waves in leads II, III, and aVF and a left axis shift (-74°) and bifascicular truncular LBBB (open arrow) with wide QRS complex (60 ms) and normal mean electrical axis (+86°) is present. At this time, cardiac troponin I concentration was 10.2 ng/mL (normal:0—0.09 ng/mL). Speed 50 mm/s and calibration 10 mm/mV. B. A 6-lead electrocardiogram 2 weeks after discharge of case 1. Notice the presence of sinus rhythm with a rate of 214 beats/min. Normal PR interval was present with a narrow QRS complex (30 ms) and a left axis shift (-56°), indicating left anterior fascicular block. At this time, cardiac troponin I concentration was 0.78 ng/mL. Speed 50 mm/s and calibration 10 mm/mV. C. A 6-lead electrocardiogram 8 months after initial discharge of case 1. Notice the presence of a sinus rhythm with a rate of 180 beats/min. Normal PR interval
The cat improved (some residual left head-tilt) and was discharged with clindamycin (15 mg/kg PO q 12 h), since we considered toxoplasmosis as a possible cause of the clinical signs. However, clindamycin was discontinued after 4 days when the results of the toxoplasmosis titers were found to be negative.
All neurologic signs resolved after one week. The cTnI concentration had decreased (0.65 ng/ mL). Six-lead electrocardiogram (ECG) revealed sinus tachycardia (250 beats/min) conducted with a post-divisional LBBB. The duration of the PR interval (60 ms) and QRS complex (40 ms) were normal. Mean electrical axis (+45°) was normal (Fig. 3B).
The cat was reevaluated approximately 2 months later, and the previously detected pulmonary mass appeared unchanged on thoracic radiographs. The cTnI concentrations were not obtained. Electrocardiogram revealed normal sinus rhythm (200 beats/min). The duration of the PR interval (60 ms) and QRS complex (40 ms) were normal. Mean electrical axis (+72°) was normal (Fig. 3C).
Case 3
A 16-year-old male-castrated Maine Coon cat (4.7 kg) was referred for bradycardia noted by the primary veterinarian, who evaluated the cat for a 1-day history of lethargy and inappetence. Previous medical history included left Horner’s syndrome and chronic kidney disease. An electrocardiogram (ECG) performed by the primary veterinarian revealed bradycardia and a single lead ECG revealed paroxysmal second-degree AV block with junctional escape rhythm at 150 beats/min (Fig. 4). Vital signs on presentation to Cornell University included a heart rate of 180 beats/min, respiratory rate of 40 breaths/min, and rectal temperature of 37 °C. A grade I/VI systolic murmur was auscultated over the sternum.
Thoracic radiographs revealed mild car- diomegaly with no pulmonary edema. Biochemistry profile revealed some abnormalities (glucose 208 mg/dL [normal: 71—159 mg/dL]; urea nitrogen 38 mg/dL [normal: 18—36 mg/dL]; potassium 3.3 mmol/L [normal: 3.5—5.8 mmol/L]). Doppler systolic blood pressure was 142 mmHg. The cTnI was present with narrow QRS complex (30 ms) and left axis shift (-56°), indicating left anterior fascicular block. This axis shift remained unchanged compared with the previous recording. At this time, cardiac troponin I concentration was 0.15 ng/mL. Speed 50 mm/s and calibration 10 mm/mV.
Figure 3 A. A 6-lead electrocardiogram at admission of case 2. Notice the presence of sinus rhythm (230 beats/ min) with first-degree atrioventricular block (PR interval progressively prolongs from 160 ms to 200 ms) and trun- cular left bundle branch block (black arrow) with wide QRS complex (60 ms) and a normal mean electrical axis (+63°).
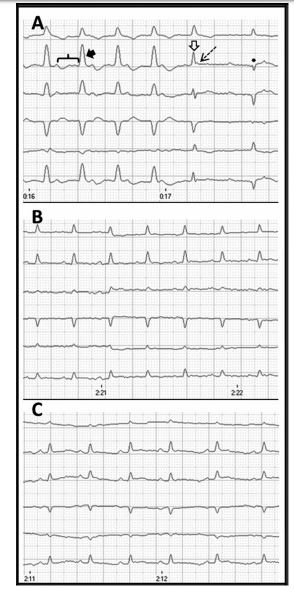
Also note the alternating post-divisional left bundle branch block (open arrow) with narrow QRS complex (30 ms) and normal mean electrical axis (+45°), and left anterior fascicular block (asterisk) with narrow QRS com¬plex (30 ms) and left axis shift (-39°). One episode of second-degree atrioventricular block is also seen (dashed arrow). At this time, cardiac troponin I was 7.55 ng/mL. Speed 50 mm/s and calibration 10 mm/mV. B. Notice the presence of sinus rhythm (250 beats/min) conducted with concentration was elevated at 0.33 ng/mL. Six-lead electro¬cardiogram (ECG) revealed sinus rhythm (176 beats/min) with first-degree AV block (PR interval: 120 ms). A wide QRS complex (60 ms) with a right axis shift (-99°) indicated RBBB (Fig. 5A). Echocardiography revealed normal cardiac structure with hyperkinetic wall motion (IVSd: 4.8 mm; LVFWd: 4.9 mm; LVIDd: 14.7 mm; LA:Ao: 1.3; FS: 72%).
No treatment was administered, and the cat was discharged, as the owner reported that the cat was no longer exhibiting clinical signs. Three weeks later, the cTnI concentration had increased (1.13 ng/mL). At this time, the ECG revealed sinus bradycardia (136 beats/min), first-degree AV block (PR interval: 100 ms), wide QRS complex (60 ms), and normal axis (+95°), suggesting the presence of truncular LBBB (Fig. 5B). The diagnosis of trifascicular block was based on the presence of alternating RBBB and truncular LBBB with first-degree AV block and paroxysmal second-degree AV block (Fig. 4).
Five weeks after initial presentation cTnI concentration, echocardiogram and 6-lead ECG were repeated. The cTnI concentration was nearly normal at 0.1 ng/mL (ref 0—0.09 ng/mL). The echocardiogram remained normal. Electrocardiogram showed sinus rhythm (140 beats/min) conducted with first-degree AV block (PR interval: 100 ms), normal QRS complex (40 ms), and normal mean electrical axis (+94°). These findings suggested a near complete resolution of conduction disturbance (Fig. 5C).
The cat continued to do well for approximately 8 months, at which time, he was euthanized due to complications associated with chronic kidney disease.
Discussion
We report the presence of trifascicular block in three cats that had concurrent elevations in cTnI concentrations. Recently, guidelines in the human literature forego the use of the term "trifascicular" a post-divisional left bundle branch block. Normal PR interval was present (60 ms) with narrow QRS complex (40 ms) and normal mean electrical axis (+45°). At this time, cardiac troponin I was 0.65 ng/mL. Speed 50 mm/s and calibration 10 mm/mV. C. Notice thepresence of sinus rhythm with a rate of 200 beats/min. Normal PR interval was present (60 ms) with narrow QRS complex (30 ms) and normal mean electrical axis (+72°). Cardiac troponin I concentrations were not obtained at this time. Speed 50 mm/s and calibration 10 mm/mV.
Figure 4 Lead II electrocardiogram before admission of case 3. The first part of the tracing shows a sinus rhythm with a heart rate of 166 beats/min, followed by a decrease of the sinus rate to 150 beats/min
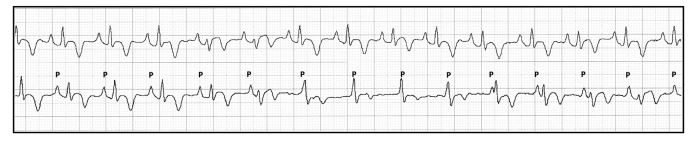
Notice the presence of advanced second-degree atrioventricular block with a junctional escape rhythm and isorhythmic atrioventricular dissociation with synchronization type 2. Speed 50 mm/s and calibration 20 mm/mV.
Figure 5 A. A 6-lead electrocardiogram at admission of case 3. Notice sinus rhythm (176 beats/min) and first-degree atrioventricular block (PR interval: 120 ms)
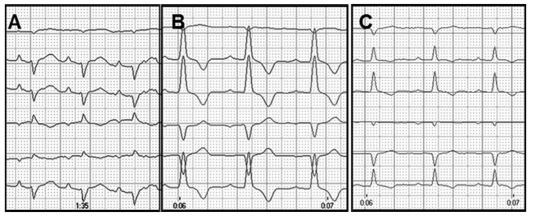
A wide QRS complex (60 ms) with right axis shift (-99°) suggests the presence of a complete right bundle branch block. At this time, the cardiac troponin I was 0.33 ng/mL. Speed 50 mm/s and calibration 10 mm/mV. B. A 6-lead electrocardiogram 3 weeks after admission of case 3. Notice sinus bradycardia with a rate of 136 beats/min and first-degree atrioventricular block (PR interval: 100 ms). Wide QRS complex (60 ms) with normal axis (+95°) suggests the presence of truncular or pre-divisional left bundle branch block. At this time, the cardiac troponin I concentration was 1.13 ng/mL. Speed 50 mm/s and calibration 5 mm/mV. C. A 6-lead electrocardiogram 5 weeks after admission of case 3. Notice sinus bradycardia (140 beats/min) and first-degree atrioventricular block (PR interval: 100 ms). Normal QRS complex (40 ms) with normal axis (+94°) suggests a near complete resolution of conduction disturbance. At this time, the cardiac troponin I concentration was 0.10 ng/mL. Speed 50 mm/s and calibration 5 mm/mV.
for terminology more specific to the affected bundles [3]. Here, we use the term trifascicular generically to implicate the severity of disease from a clinical standpoint. Affected bundles have been specified in each case.
Proper conduction of the ventricular impulse is dependent on the AV node and its communicating intraventricular fascicles. Beneath the AV node, the intraventricular conduction system branches into three fascicles; one right, and two (anterior and posterior) left fascicles. The left anterior fascicle travels superficially along the left ventricular outflow tract, where it is positioned anteriorly and superiorly. The left posterior fascicle is located within the mitral inflow tract. The trifascicular theory, first described in humans in 1968 , remains the most accurate anatomical description of the conduction system [4,5].
Lesions affecting the fascicles may lead to varying degrees of blocked conduction (Fig. 6). Monofascicular blocks include, separately, RBBB, LAFB, and left posterior fascicular block. Bifascicular blocks include 1) the simultaneous block of right bundle branch with either the left anterior or posterior branch, or 2) the simultaneous block of both left fascicles (also called 'truncular’ or 'predivisional block’, interchangeably). Trifascicular block is the most severe and occurs when both left and right bundle branches are interrupted due to infranodal disease (true trifascicular block; Case 2), or when a bifascicular block accompanies a nodal block with evidence of AV conduction disturbance (cases 1 and 3; Fig. 6) [4,5]. In the reported cases, an assumption of infranodal block is made; however, it is important to note that the distinction between infra- and AV-nodal block cannot be made without electrophysiological mapping studies.
Figure 6 Models display varying degrees of conduction system block. AVN: atrioventricular node; HB, His bundle; TLB, truncular region of the left bundle; AF, anterior fascicle of the left bundle; PF, posterior fascicle of the left bundle; RBB, right bundle branch; MEA, mean electrical axis; RBBB, right bundle branch block; LPFB, left posterior fascicle block; LAFB, left anterior fascicular block; AVB, atrioventricular block.
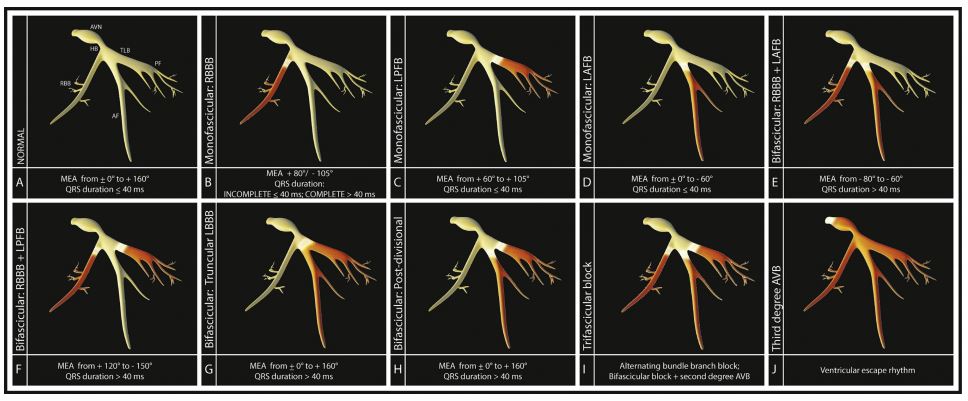
Intraventricular conduction disturbances are primarily diagnosed by electrocardiography in both humans and domestic animals. Whereas types of monofascicular or bifascicular block can be identified by specific ECG patterns, diagnosis of trifascicular block can be more difficult to confirm in the absence of electrophysiological mapping studies [6,7]. A definitive electrocardiogram (ECG) diagnosis of trifascicular block is made if a single ECG recording reveals, at separate times, blocks at each of the three branches of the AV bundle. Alternatively, one may suspect trifascicular block if bifascicular block is present in concordance with delayed conduction below the onset of the AV node. It is important to note that a prolonged PR interval (first-degree AV block) may represent a conduction delay at the level of the AV node, instead of the level of the intraventricular fascicles. Therefore, true trifascicular disease should not be considered unless bifascicular block is present along with second-degree AV block [5,8—10].
Conduction disturbances at the level of the fascicles can be caused by a variety of disorders. In humans, idiopathic bundle branch fibrosis is most commonly reported on necropsy (estimated at 38%), though myocarditis, amyloidosis, congenital abnormalities, and atherosclerotic coronary artery disease together comprise a similar statistic [11]. Trifascicular block has been reported in the dog [12] and is associated with a similar variety of diseases [12—14]. Advanced second-degree and third-degree AV block, as well as bifascicular block, have been reported in the cat [15—18].
Based on the elevated cTnI concentrations in these cases, acute myocarditis was suspected as the inciting cause of trifascicular block [19]. The prevalence of myocarditis-related conduction block in cats (and dogs) is unknown. The mecha¬nism by which myocarditis leads to abnormalities of the conduction system has been previously described in dogs [20,21]. In these cases, severe lymphocytic and plasma cell infiltrates, as well as loss of conduction fibers, were noted within the AV-nodal tissues, suggesting this infiltrative process results in damage and decreased function of the conduction system. In situations of resolving conduction block, the presence of a gradually resolving interstitial edema may explain improvement of conduction over time [22].
No definitive cause for cardiac conduction abnormalities was found in the described cats, though acute myocarditis was considered likely. Cardiac cell death was evidenced by the elevated cTnI concentration in each case. Myocarditis has been reported in the cat secondary to Toxoplasma gondii [23], Bartonella henselae [24], and Streptococcus canis [25]. Other, as-yet-undetermined, agents may also contribute to the disease, with definitive diagnoses hindered by the difficulties obtaining myocardial biopsies from felines. Autoimmune disease or paraneoplastic syndromes were considered unlikely as clinical signs resolved without specific therapy. Owing to the wide variation in clinical presentation between cases, different inciting agents for each case cannot be ruled out.
The ECG changes comprising the trifascicular block varied among the three cats. It is unknown whether these changes were specific to different disease processes, or were simply representative of different stages of the same disease. Recovery of normal conduction appeared to follow a similar course, as the LAFB was documented to be the last remaining blocked fascicle in two of the three cats.
In conclusion, herein we reported three cats with varied presentations of trifascicular block, all of which resolved spontaneously with supportive care. These cats remained alive with no regression of the cardiac rhythm for months after initial diagnosis, suggesting a good short-term prognosis.
Conflict of Interest Statement
The authors do not have any conflicts of interest to disclose.
References
- Boon JA. Appendix four. In: Boon JD, editor. Veterinary Echocardiography. Ames:Wiley-Blackwell; 2011. p. 569—79.
- Kittleson MD, Kienle RD. Electrocardiography: basic concepts, diagnosis of chamber enlargement, and intraventricular conduction disturbances. In: Kittleson MD, Kienle RD, editors. Small Animal Cardiovascular Medicine. St Louis: Mosby; 1998. p. 72—94.
- Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the american heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the american College of cardiology foundation; and the heart rhythm society. Endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol 2009;53:976-81.
- Rosenbaum MB, Elizari MV, Kretz A, Taratuto ALl. Anatomical basis of AV conduction disturbances. Geriatrics 1970;25:132-44.
- Elizari MV, Acunzo RS, Ferreiro M. Hemiblocks revisited. Circulation 2007;115:1154-63.
- Dhingra RC, Wyndham C, Amat-y-Leon F, Denes P, Wu D, Sridhar S, Bustin AG, Rosen KM. Incidence and site of atrioventricular block in patients with chronic bifascicular block. Circulation 1979;59:238-46.
- Krongrad E. Trifascicular block (lett). Circulation 1979;59:420.
- Femenia F, Cuesta A, Mauricio A. A rare form of trifascicular block with intermittent complete atrioven¬tricular block in a patient with Chagas disease. Cardiol J 2009;16:582-4.
- Karanikis P, Korantzopoulos P, Kountouris E, Dimitroula V, Patsouras D, Pappa E, Siogas K. Kearns-Sayre syndrome associated with trifascicular block and QT prolongation. Int J Cardiol 2005;101:147-50.
- Peters RW, Scheinman MM, Dhingra R, Rosen K, McAnulty J, Rahimtoola SH, Modin G. Serial electrophysiologic studies in patients with chronic bundle branch block. Circulation 1982;65:1480-5.
- Waller BF, Gering LE, Branyas NA, Slack JD. Anatomy, histology, and pathology of the cardiac conduction system-Part V. Clin Cardiol 1993;16:565-9.
- Baron Toaldo M, Critelli M, Santilli RA. ECG of the month. Trifascicular block causing syncope. J Am Vet Med Assoc 2011;239:438-40.
- Billen F, Van Israel N. Syncope secondary to transient atrioventricular block in a German Shepherd Dog with dilated cardiomyopathy and atrial fibrillation. J Vet Cardiol 2006;8:63-8.
- Schrope DP, Kelch WJ. Signalment, clinical signs, and prognostic indicators associated with high-grade second- or third-degree atrioventricular block in dogs: 124 cases (January 1, 1997- December 31, 1997). J Am Vet Med Assoc 2006;228:1710-7.
- Kellum HB, Stepien RL. Third degree atrioventricular block in 21 cats. J Vet Intern Med 2006;20:97-103.
- Penning VA, Connolly DJ, Gajanayake I, McMahon LA, Luis Fuentes V, Chandler KE, Volk HA. Seizure like episodes in 3 cats with high-grade atrioventricular dysfunction. J Vet Intern Med 2009;23:200-5.
- Nakamura RK, Zimmerman SA. ECG of the Month: second- degree atrioventricular block in a cat. J Am Vet Med Assoc 2012;15(241):433-4.
- Machida N, Nakamura T, Kiryu K. Cardiopathological observation of bifascicular block in an aged cat. J Vet Med Sci 1992;54:391-3.
- Trafney DJ, Oyama MA, Wormser C, Reynolds CA, SingletaryGE, Peddle GD. Cardiac troponin-I concentrations in dogs with bradyarrhythmias before and after artificial pacing. J Vet Cardiol 2010;12:183-90.
- Church WM, Sisson DD, Oyama MA, Zachary JF. Third degree atrioventricular block and sudden death secondary to acute myocarditis in a dog. J Vet Cardiol 2007;9:53-7.
- Kaneshige T, Machida N, Nakao S, Doiguchi O, Katsuda S, Yamane Y. Complete atrioventricular block associated with lymphocytic myocarditis of the atrioventricular node in two young adult dogs. J Comp Pathol 2007;137:146-50.
- Morimoto S, Kato S, Hiramitsu S, Uemura A, Ohtsuki M, KatoY, SugiuraA, Miyagishima K, Yoshida Y, Hishida H. Role of myocardial interstitial edema in conduction disturbances in acute myocarditis. Heart Ves 2006;21:356-60.
- Simpson KE, Devine BC, Gunn-Moore D. Suspected toxoplasma-associated myocarditis in a cat. J Feline Med Surg 2005;7:203-8.
- Varanat M, Broadhurst J, Linder KE, Maggi RG, Breitschwerdt EB. Identification of Bartonella henselae in 2 cats with pyogranulomatous myocarditis and dia¬phragmatic myositis. Vet Pathol 2012;49:608-11.
- Matsuu A, Kanda T, Sugiyama A, Murase T, Hikasa Y. Mitral stenosis with bacterial myocarditis in a cat. J Vet Med Sci 2007;69:1171-4.
^Наверх









