A technique for in vitro culture of canine valvular interstitial cells

Author information
Heaney A.M., Bulmer B.J., Ross C.R., Schermerhorn T. A technique for in vitro culture of canine valvular interstitial cells // J Vet Cardiol. 2009 Jun;11(1):1-7.
Abstract
OBJECTIVE: To develop a method for in vitro culture of canine valvular interstitial cells (VICs).
ANIMALS, MATERIALS AND METHODS: Canine VICs were isolated from the distal third of the anterior mitral valve leaflet using an explant technique and maintained in cell culture. Molecular phenotyping of the cultured cells was performed using reverse transcription polymerase chain reaction and immunocytochemistry.
RESULTS: Cells resembling fibroblasts migrated from canine mitral valve explants and were maintained in culture for up to eight passages. Establishment of the valve explant required collagen but once established, subsequent passages grew on non-coated plastic plates. At confluence the cultured cells exhibited the characteristic whorled pattern of fibroblasts in culture. The isolated valve cells expressed vimentin but not platelet endothelial cell adhesion molecule or von Willebrand's factor, consistent with the molecular phenotype of VICs.
CONCLUSIONS: VICs can be readily isolated from canine mitral valve leaflets and successfully maintained in culture using standard culturetechniques. The described techniques permit the study of bioactive VICs in a controlled environment and may be a useful in vitro model for investigation of cellular and molecular alterations associated with canine chronic degenerative valve disease.
Introduction
Chronic degenerative valve disease (CDVD) is the most common acquired cardiac disease in the domestic canine population.1-3 This disease results in predominantly mitral valve regurgitation due to myxomatous degeneration of the mitral valve and subsequent left-sided volume overload and eventually congestive heart failure. Valvular interstitial cells (VICs) are the most prevalent cell type in cardiac valves but to date they have not been extensively studied in the dog.4 Interstitial cells are responsible for synthesizing the extracellular matrix (collagen, elastin, and proteoglycans) and mediating the ongoing repair and remodeling which provides valvular durability.5 The phenotype of VICs is known to be heterogenous and demonstrate different cell morphology and immunohistochemical staining characteristics depending on the age of the cell and location within the valve.4 Mulholland and Gotlieb reviewed the cell biology and heterogeneity of VICs but it was only with later studies that these different cell types were related to alterations in function (normal versus activated interstitial cells).5,6 Activated VICs are found in fetal human and sheep valves as well as in valves with evidence of myxomatous degeneration, suggesting that during times of disease and repair these cells revert to a more fetal-like state.5
In vitro studies have demonstrated that VICs possess contractile properties and serve complex roles within the valve structure beyond merely providing passive structural support.6 VICs are embedded in a three-dimensional matrix in vivo, where they function to secrete matrix and engage in cellular and molecular interactions with the matrix material.4 Valvular interstitial cells are capable of synthesizing collagen, elastin, proteoglycans, fibronectin, growth factors, cytokines, chemokines as well as matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs).7
Many studies have been performed on cultured VICs in various species but a culture technique has not previously been described for canine valves. Because of the prevalence of CDVD in the dog as well as recent support for the dog as a naturally occurring model of human mitral valve prolapse, studying VICs in this species could benefit both dogs and humans with valvular disease. The purpose of this study was to develop a culture technique that could reliably culture canine VICs and to evaluate the utility of coating the culture plates with collagen I in canine VIC culture.
Animals, materials and methods
Valve tissue source
After owner consent, normal canine mitral valve tissue was obtained from dogs at the time of necropsy. Immediately after harvesting, the tissues were processed for cell culture. Canine kidney tissue was also harvested from a client- owned dog with consent immediately after euthanasia for reasons unrelated to the study and was used as a positive control for the presence of endothelial cells.
Cell culture method
The cells were prepared using a method modified from that described by Katwa et al.8 and Lester et al.9 Briefly, the distal one-third of the anterior mitral valve leaflet was harvested from dogs undergoing necropsy within 36 h of death. The samples were immediately placed in cold phosphate buffered saline (PBS) and maintained on ice until prepared for culture. For culture, the endothelial surface of the valve was abraded with a scalpel blade and the tissue was cut into approximately 1 mm square sections. Each 1 mm2 section of the valve sample was placed into an individual well of a non-coated or collagen-coated 6-well plastic culture plate. The plates were coated with filtered collagenf diluted with phosphate buffered saline and 5M NaOH titrated to achieve a pH of 7.0 with HCl. The filtered collagen solution was used to thinly coat the wells of the plate and the plates were incubated for 1 h at 37 °C with 95% O2 and 5% CO2. The coated plates were then placed in the cell culture hood overnight to dry. The plates were sealed and stored at 4 °C until use. Prior to use, collagen-coated plates were rehydrated by rinsing each well with sterile water. All samples were covered with one drop of Dulbecco’s Modified Eagle’s Medium (DMEM) with 20% fetal bovine serum (FBS) and 1% antibiotic/ antifungal. After 12 h, 1 ml of media was added to each well and the media was subsequently changed every 3—4 days.
f Bovine collagen, Type I, BD Biosciences, Bedford, MA, USA.
Figure 1 Photograph taken 4 days after tissue was placed in a 6-well culture plate showing fibroblast-like cells migrating from the valve tissue in culture
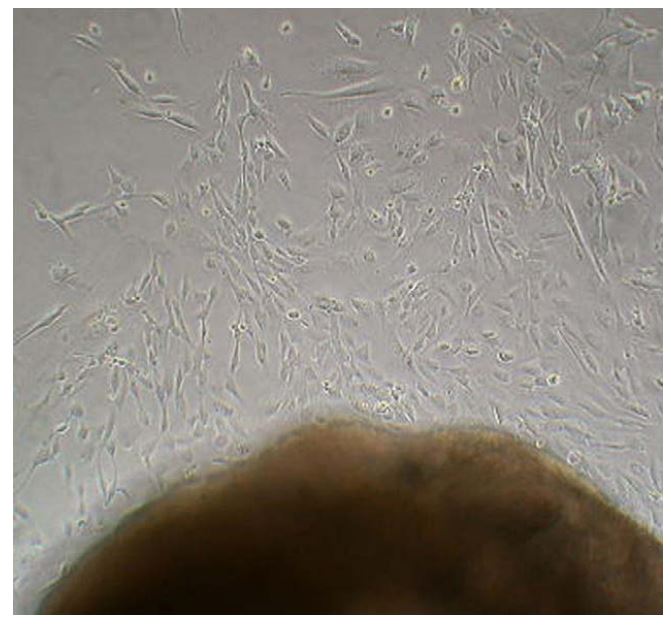
At this non-confluent state the cells are multipolar and spread out on the plate.
The original 1 mm2 tissue block was removed once a colony of explanted cells developed (Fig. 1). When the cells were 75—100% confluent they were passaged using standard trypsinization techniques. This technique involved removing the media from each well, replacing it with 1 ml of 0.25% trypsin for 5—8 min (until cells began to release from the plate), and then adding 1 ml of media to the plate while pipetting up and down to release the remaining cells. Once cells were released from the plate, the suspended cells were placed in a 15 ml conical tube and centrifuged for 5 min at 1050 RPM with a slow acceleration and deceleration. The supernatant was discarded and the pellet of cells was resuspended in culture media. The cells were split 1:4 and re-plated into either new 6-well plates or T-75 flasks, depending on the intent of use, and maintained in the DMEM with 20% fetal bovine serum (FBS) and 1% antibiotic/antifungal. Cells from each passage were placed in freeze media (the above growth media with 7.5% dimethyl sulfoxide added) and stored in liquid nitrogen.
Phenotypic characterization
Reverse transcription polymerase chain reaction (RT-PCR) protocol
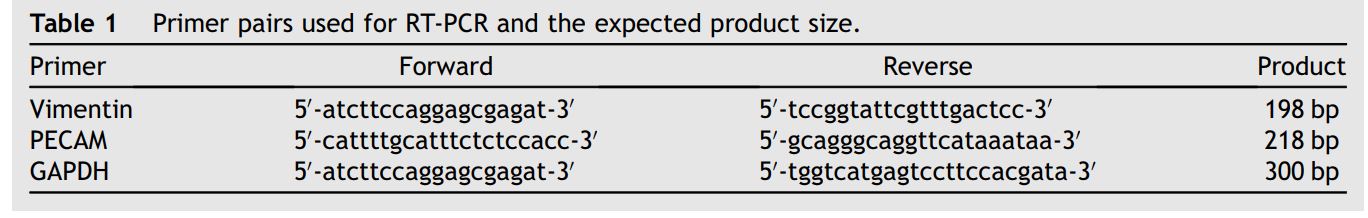
Primers for vimentin, platelet endothelial cell adhesion molecule (PECAM), and glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) were used as listed in Table 1. Vimentin primers were developed based on a known rat vimentin sequence (GenBank Accession number X62952) using Primer3.10 The canine genome had not been released when these studies were performed therefore canine specific primers were not used. Primers for PECAM were based on the human RNA sequence.11 GAPDH primers were employed as a positive control. Total RNA was prepared from cells using Tri Reagent RNA Isolation Reagent using phenol:- chloroform:isoamylalcohol extraction as recommended by the manufacturers.g Canine kidney total RNA was isolated with the same method to serve as a positive control for PECAM.
Amplification of vimentin and PECAM mRNA by RT-PCR was performed in a TGradient Thermocy- clerh with a mixture (total reaction volume was 25 ml) that included: Access Quick Mastermix* i (12.5 mL), 1 ml of each specific oligonucleotide primer, total RNA (3 ml), AMV Reverse Transcriptase (0.5 ml), and filtered dH2O (q.s. to 25 ml). The mixture was incubated for 45 min (min) at 48 °C and then at 95 °C for 1 min and 15 s. For amplification the mixture was cycled 30 times through the following three conditions: 95 °C for 45 s, 55 °C for 45 s, and 72 °C for 1 min and 15 s. The reaction was terminated by heating the reaction to 72 °C for 5 min and then the reaction was held at 4 °C. Products from the PCR reaction were resolved by electrophoresis at 75 V for 30—45 min on a 1.5% agarose gel and detected with ethidium bromide staining and UV light. Products were isolated from the agarose gel and the identity of the PCR products was confirmed by sequence analysis performed using an automated DNA analyzer.j,k
Immunocytochemistry protocol Immunocytochemistry was performed on cytospun preparations of cultured VICs. For cytospin preparations, confluent VICs maintained in a T-75 flask were detached by brief trypsinization, washed and resuspended in PBS. Aliquots of VIC suspensions (200 ml) were placed into individual wells of a Shandon Cytospin® cytocentrifuge. The samples were spun at 700 RPM for 3 min onto a glass slide. Slides were air-dried for at least 4 h prior to fixation and staining for vimentin and factor VIII.
g Sigma—Aldrich, St. Louis, MO, USA. h Biometra, Goettingen, Germany.
i AccessQuick™ RT-PCR System, Promega, Madison, WI, USA. j DNA Gel Extraction Kit, Millipore Corporation, Bedford, MA, USA.
k ABI 3700 and ABI 3730 analyzers, KSU Dept Plant Pathology, Manhattan, KS, USA.
Table 1 Primer pairs used for RT-PCR and the expected product size
Vimentin protocol
Primary antibody was applied [Vimentin prediluted by manufacturer (DAKO)] by hand and incubated. The slide was rinsed and biotinylated IgG was applied and incubated. The slide was rinsed again and avidin-alkaline phosphatase was applied and incubated. After rinsing, Enhancer was applied and incubated followed by Fast Red A and Naphthol. Fast Red B was then applied and incubated and the slide rinsed again. Hematoxylin counterstain was added followed by Bluing Reagent as a post counterstain. The slide was then rinsed and dehydrated by three changes in 100% EtOH and two changes in Xylene followed by placement of a cover slip.
Factor VIII protocol
Primary antibody was applied [Factor VIII diluted 1:400 (DAKO)] by hand and incubated. After rinsing, avidin-HRPO was added to the slide. The slide was rinsed and DAB and DAB H2O2 were applied and incubated. After rinsing again, one drop copper was added and incubated. Following another rinse, the slide was incubated with Hematoxylin as a counterstain. The slide was rinsed again prior to incubating with Bluing Reagent as a post counterstain. The final rinse and the dehydration steps were performed as for the vimentin slides.
Results
Evaluation of collagen-coated plastic wells
Nine anterior mitral valve leaflet samples were plated on plastic (non-coated) wells and 8 anterior mitral valve leaflet samples were plated on collagen-coated plastic wells. No growth was noted for the 9 mitral valves that were plated without collagen. Of the 8 canine mitral valves plated on collagen-coated plates, 6 of these 8 samples (75%) successfully cultured mitral VICs. The two sets of valves that were unsuccessful initially adhered to the plate, but both samples were contaminated within 2—7 days. Canine valvular interstitial cells from the mitral valves were repeatedly cultured and passaged. Cells stored in liquid nitrogen were cultured with most cells surviving the stored time period. It took nearly a week in the culture environment after being removed from liquid nitrogen for the cells to regain their initial appearance in culture and begin to undergo cell division.
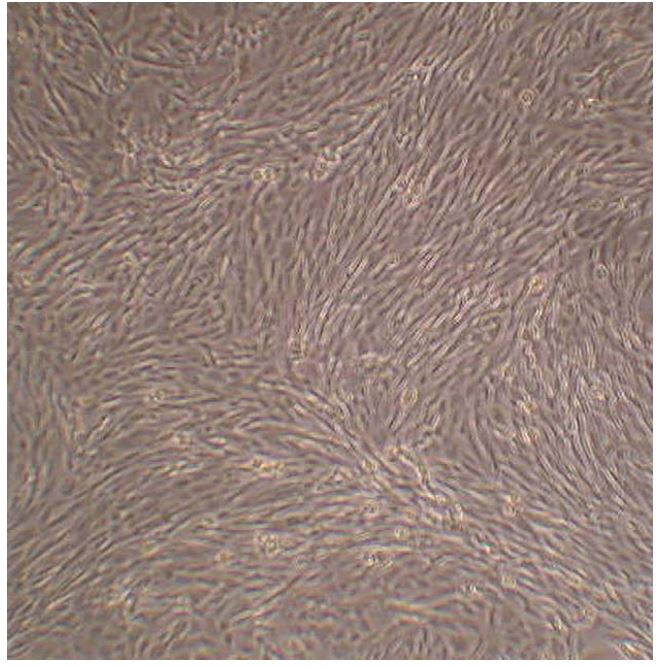
When cells were not confluent they appeared multipolar and well spread out on the culture surface. Once confluency was achieved they became more cobblestone in appearance and developed the whorling pattern characteristic of fibroblast-like cells (Fig. 2). The finding of a mixed population of VICs in culture displaying both a fibroblast-like whorling as well a more cobblestone-like appearance has been appreciated by other investigators. This has been attributed to the range of phenotypes of VICs.12
Figure 2 Photograph taken as cells reached confluency. Note the whirling pattern that is characteristic of fibroblast-like cells
Phenotyping results
Vimentin mRNA expression by canine valvular interstitial cells was confirmed using RT-PCR (Fig. 3). RT-PCR using vimentin primers yielded a 131 bp product from cultured VICs. The nucleotide sequence of the RT-PCR product (GenBank# EF432321) is identical to sequence contained within a 783 bp partial primary sequence of canine vimentin (Gen Bank# DR103772) and a 1906 bp predicted canine vimentin sequence (GenBank # XM535175). Immunocytochemistry revealed strong positive staining for vimentin by all cells (Fig. 4).
The canine VICs were negative for PECAM and von Willebrand’s expression. In RT-PCR experiments, PECAM mRNA was detected in canine kidney used as control for detection of endothelial cells, but was not detected in canine VICs. Von Willebrand’s immunocytochemistry of cultured VICs showed only scant staining that was similar to the background observed in negative controls. Overall, the RT-PCR and immunocytochemistry results confirmed that the cells cultured from canine valves contained abundant amounts of the intermediate filament vimentin, as expected for VICs, but lack PECAM and Factor VIII expression indicating these cells are not of endothelial origin.
Figure 3 RT-PCR performed using total RNA from cultured cells (VICs) and from total RNA harvested from homogenized canine kidney (DK)
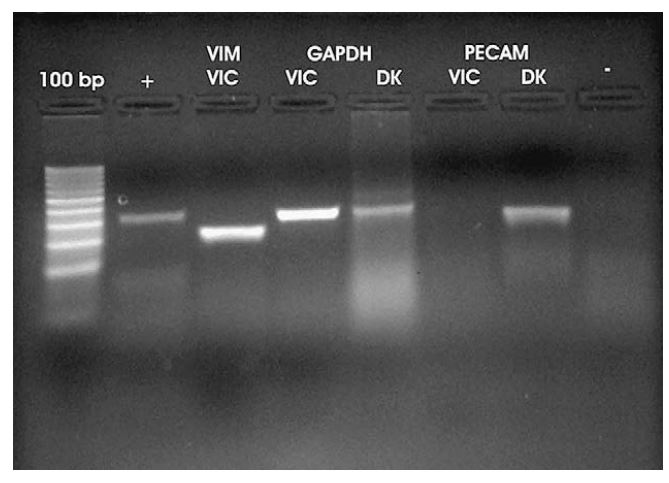
From left to right: Lane 1 is positive control RNA supplied by manufacturer with primers to amplify product of 320 bp. Lane 2 is vimentin (VIM) using primers to amplify 198 bp product. Lanes 3 and 4 show GAPDH utilized as an internal control with primers meant to amplify 300 bp present in both cultured cells (VICs) and kidney tissue (DK). In lanes 5 and 6 PECAM is included to rule out the presence of endothelial cells. PECAM is expressed in canine kidney (DK) but not in VICs. Lane 7 is a negative control using vimentin primers with proposed VICs without transcriptase.
Figure 4 Immunocytochemistry performed with primary antibody against vimentin
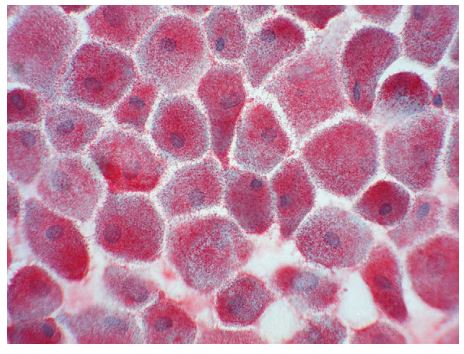
All cells stained heavily for this intermediate filament.
Discussion
In this study, a simple protocol was developed for in vitro culture of canine VICs. The described technique allows direct study of canine VICs and will facilitate future studies to elucidate the role of VICs in canine valvular health and disease. Studies using the cultured VIC system have great potential to advance our understanding of canine mitral valve physiology and its response to injury. Exposure of vasoactive agents to VICs could enlighten the veterinary cardiology community as to the changes these agents can cause with regard to collagen production and alterations in MMP and TIMP concentrations. The results of these studies could be pivotal in understanding the etiology and pathogenesis of mitral valve disease. Because of the possibility of shear stress on the valve initiating or perpetuating CDVD, exposure of the VICs to shear stress would reveal VICs response to such a stressor. Co-culture with endothelial cells could promote further understanding of the interaction between these two cell populations under a variety of stressors. These are merely examples of a multitude of studies that could be performed using this cell culture technique.
As described, establishment and maintenance of VICs in cell culture are relatively simple. However, the study identified one step that appears crucial for success. Although fibroblastlike cells, such as VIC, are typically strongly adherent to plastic culture vessels, the present study found that pre-coating culture wells with collagen was very important to establish the VIC culture. However, once the VICs have migrated from the valve tissue and reached confluence, subsequent passages do not require collagen for efficient culture. This observation suggests that the collagen coating is more important for keeping the valve tissue in place and close to the surface of the culture well in order to permit VICs to migrate from the tissue and is not a requirement for the VIC culture per se.
As previously noted, the molecular phenotype of cardiac valve interstitial cells is variable, which presented a unique challenge for establishing whether the phenotype of cells grown from canine mitral valves were VICs. Vimentin expression was selected as the positive VIC marker because it is expressed by all VIC phenotypic variations. Because endothelial-derived cells comprise a portion of the cell types found in the canine mitral valve, endothelial marker expression by the cultured cells was assessed. Expression of PECAM and Factor VIII, both indicators of endothelial cell origin was used as negative control in RT-PCR and immunocytochemical studies. The cultured valve cells were always negative for both endothelial markers, indicating the cells were not of endothelial lineage. The results of phenotyping support the conclusion that the cultured cells are VICs but the isolation and culture techniques used make it unlikely that anything but VICs would be cultured. One reason is that only VICs and endothelial cells have the potential to migrate from the valve tissue. A second reason is that the growth environment used in the described technique is unlikely to support endothelial cell growth.13 The morphology of the isolated cells and the growth characteristics of the cells in culture were consistent with VICs, which typically appear and grow as fibroblast-like cells. Gross and microscopic observations of cell morphology and growth combined with the results of molecular phenotyping confirm that the cells cultured via this technique were VICs. As with many small scale investigations there were numerous limitations in the present study. One limitation of the explant technique is that it selects for VICs that are most capable of migration and the cultured cells therefore may not represent the spectrum of VICs in the valve tissue. As previous studies have shown a high degree of plasticity in VICs, it seems likely that given the correct environment all VICs have the potential to become motile.6,12 The ease of reproducing this technique allows for populations of VICs from all mitral valve tissue cultured as long as strict aseptic technique is performed. In our opinion, the benefit of the reproducibility of the technique far outweighs any potential selection for more motile cells.
The authors also recognize there are other studies that should be done to further characterize these cells in culture. Determining the amount of alpha smooth muscle actin present would be valuable in differentiating fibroblasts from myofibroblasts (activated valvular interstitial cells) further enabling characterization of the cultured cell population. Despite the use of similar explant techniques in other species to effectively culture a pure population of valvular interstitial cells, the presence of other cells should be excluded.8,9 In addition to confirming the absence of endothelial cells as has been described in this manuscript and despite the low probability of other cell lineage growth given the culture technique and conditions, markers to exclude other cell populations should be investigated. Using a marker of smooth muscle cells to prove their absence in this population should be performed by using calponin or an alternate marker of smooth muscle.
Another useful future investigation could be to determine how many passages it takes for the cells to reach senescence to determine the optimal time when experiments should be performed on these cells. Because of their plasticity determining the presence of alpha smooth muscle actin over passages would be useful to document if the VICs undergo transformation from fibroblast to myofibroblast in the culture environment which could alter the reproducibility and results of studies. A limitation of data obtained using cell culture is the possibility that it oversimplifies the in vivo system. While this concern must be addressed when using the canine VIC model to study CDVD, the ability to study VICs under multiple experimental conditions easily and inexpensively without the ethical concerns of using live animals makes the canine VIC model an attractive research option.
Conclusions
We have developed a reliable protocol for isolation and maintenance of canine VICs in culture. The described methods can generate large populations of VICs from canine valve tissue for use in cellular and molecular studies. Canine VICs may serve as a useful model to elucidate the role of VICs in CDVD and other canine cardiac valve disorders.
Acknowledgements
This study was funded by the Waltham Foundation, ACVIM Cardiology Resident Research Grant and the Kansas State University Clinical Resident Research Grant.
References
- DetweilerDK, Luginbuhl H, Buchanan JW, Patterson DF. The natural history of acquired cardiac disability in the dog. Ann N Y Acad Sci 1968;147:318-329.
- Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci 1965;127: 481-516.
- Pensinger RR. Comparative aspects of mitral valve disease in dogs. Ann N YAcad Sci 1965;118:525-534.
- Durbin AD, Gotlieb AI. Advances toward understanding heart valve response to injury. Cardiovasc Pathol 2002;11:69-77.
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001;104:2525-2532.
- Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol 1996;12:231-236.
- Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. Cells in focus: the cardiac valve interstitial cell. Int J Biochem Cell Biol 2003;35:113-118.
- Katwa LC, Ratajska A, Cleutjens JP, Sun Y, Zhou G, Lee SJ, Weber KT. Angiotensin converting enzyme and kininase-II- like activities in cultured valvular interstitial cells of the rat heart. Cardiovasc Res 1995;29:57-64.
- Lester W, Rosenthal A, Granton B, Gotlieb AI. Porcine mitral valve interstitial cells in culture. LabInvest 1998;59:710-719.
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press; 2000. p. 365-386.
- Sauer S, Lehrach H, Reinhardt R. MALDI mass spectrometry analysis of single nucleotide polymorphisms by photocleavage and charge-tagging. Nucleic Acids Res 2003;31:e63.
- Blevins TL, Carroll JL, Raza AM, Grande-Allen KJ. Phenotypic characterization of isolated valvular interstitial cell subpopulations. J Heart Valve Dis 2006;15:815-822.
- Marin V, Kaplanski G, Gres S, Farnarier C, Bongrand P. Endothelial cell culture: protocol to obtain and cultivate umbilical endothelial cells. J Immunol Methods 2001;254: 183-190.
^Наверх









