A case of sustained atrial fibrillation in a cat with a normal sized left atrium at the time of diagnosis

Author information
J Vet Cardiol. 2005 Nov;7(2):137-42. A case of sustained atrial fibrillation in a cat with a normal sized left atrium at the time of diagnosis . Connolly D.J.
Abstract
This case illustrates an unusual presentation of atrial fibrillation in a 10-year-old male neutered Maine coon. At the time of diagnosis of the arrhythmia the size of the left atrium determined by echocardiography was within normal limits and no structural or functional heart or other systemic disease was identified. Traditionally it has been suggested that the atria must be of a sufficient size in order to sustain atrial fibrillation (multiple wavelet theory) and therefore only cats with significant cardiac disease can attain sufficiently large atria to sustain this arrhythmia. To the author's knowledge this is the first detailed case report of sustained atrial fibrillation in a cat with a normal sized left atriumand no obvious structural heart disease seen on cardiac ultrasound.
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias recognized in veterinary cardiology and can have a significant clinical impact in the small animal patient.1 Atrial fibrillation (AF) occurs most often in conditions associated with atrial dilation and cats with AF have significant left atrial or biatrial enlargementsecondary to hypertrophic, dilated or restrictive cardiomyopathy.1,2 In fact AF has been considered an end-stage event in cats with myocardial disease resulting in marked atrial enlargement.3 This report describes a cat with atrial fibrillation (AF) and a normal sized left atrium and no obvious structural or functional heart disease at the time of presentation.
Case history
A 10-year-old neutered male Maine coon with a history of tachycardia and irregular rhythm detected on routine pre-vaccination examination was referred for cardiac evaluation. The cat was not showing signs of cardio-respiratory compromise.
Figure 1 Lead II of a six lead paper trace ECG (20 mm/mV, 50 mm/s) with the cat in right lateral recumbency revealed variable R to R intervals and no visible P waves.
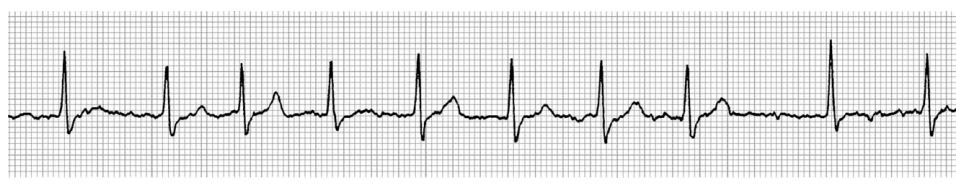
These findings are consistent with atrial fibrillation. The heart rate varied from 110 to 220 bpm.
On presentation the only significant finding was the presence of an irregular heart rhythm with associated pulse deficits. The heart rate was about 190 bpm and the pulse rate, although difficult to determine accurately, was estimated to be about half the auscultated heart rate. A thyroid goitre was not palpated and auscultation of the lung fields was unremarkable.
Diagnosis
A six lead paper trace ECG with the cat in right lateral recumbency revealed variable R wave to R wave intervals and no visible P waves consistent with AF. The heart rate varied between 180 and 200 bpm (Fig. 1). A complete blood count and biochemistry analysis were found to be within normal limits. Serum total T4 concentration was 25 nmol/l (normal range 19—65) and serum cardiac troponin I (cTnI) concentration was <0.2 mg/l (normal value < 0.2), ruling out hyperthyroidism and significant active myocardial cell damage. Thoracic radiography (dorsoventral and lateral views) was unremarkable apart from mild dilation of the aortic arch, which was considered to be an age related change. There were no radiographic signs of congestive heart failure. Systolic blood pressure analysis using the Doppler technique (an average of six readings taken from the metacarpal artery) was within normal limits at 160mmHg (normal < 175mmHg).4 Two-dimensional echocardiography from multiple views was unremarkable and no structural heart disease was evident. The left and right atrium had a normal appearance and size. The left atrial to aortic diameter ratio (taken from the right parasternal short axis view at the level of the aortic valve leaflets) was calculated from three separate images and gave a mean value of 1.26 (Fig. 2). At this institution a ratio of 1.5 or less is considered normal.5 The left atrium was also assessed from the right parasternal long axis view and measured 20 mm from the mitral valve to the apex and 16 mm from the free wall to the interatrial septum. M-mode measurements confirmed normal cardiac dimensions and the fractional shortening was at the low end of normal at 35%, probably a consequence of the tachyarrhythmia. Color Doppler analysis identified a small tricuspid insufficiency jet, the velocity of which was within normal limits. Systolic anterior motion of the mitral valve was not evident. The A and Ar waves associated with atrial contraction were not seen on Doppler examination of mitral inflow or pulmonic venous flow, respectively, and movement of the anterior mitral valve leaflet associated with atrial contraction was not evident on M-mode imaging. Abdominal ultrasound was performed in an attempt to find a systemic cause for the arrhythmia such as neoplasia and was unremarkable.
A diagnosis of atrial fibrillation without a detectable underlying cause was made and because of the sustained tachycardia the cat was treated with the b-blocker atenolol, 0.2 mg/kg once daily.
Figure 2 Right parasternal short axis view at the heart base taken at the onset of mechanical systole (just prior to opening of the aortic valve leaflets) showing a normal sized left atrium.
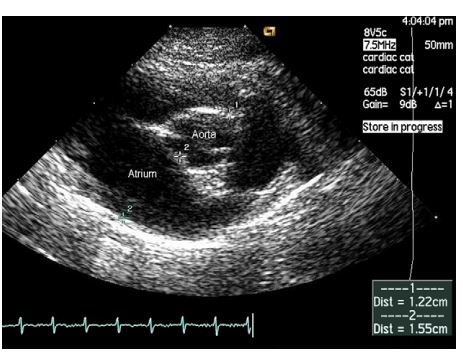
The left atrial to aortic diameter ratio in this image is 1.27.
Follow up
The cat was reassessed after 1 month, the history and physical examination findings were unchanged. A repeat ECG confirmed the presence of AF with a heart rate ranging from 140 to 180 bpm and this reduction was attributed to b blockade. Intermittent wide and bizarre QRS complexes of variable configuration were also identified which probably represents depolarisations originating in the ventricular myocardium, however, intermittent aberrant conduction of supraventricular depolarisations cannot be ruled out. Serum biochemistry, cTnI concentration and blood pressure were again within normal limits. The atria appeared dilated on echocardiography and the left atrial to aortic diameter ratio on this occasion had a mean value of 1.84. To ensure consistency all the echocardiographic examinations in this case report were performed by the author. The left atrium, measured from the long axis view had increased to 24.5 mm by 18 mm. M-mode measurements were again within normal limits and on two-dimensional echocardiography no sign of structural heart disease was evident. Doppler analysis of diastolic function was unaltered. In addition to the atenolol at the previous dose, benazepril was added at a dose of 0.5 mg/kg once daily. In view of the atrial enlargement the owner was given furosemide tablets to be used in an emergency at a dose of 2 mg/ kg prior to seeking veterinary attention.
On re-examination 1 month later history, physical examination findings and blood test results were unaltered. Electrocardiography revealed atrial fibrillation (AF) with a heart rate of between 140 and 170 bpm. However, the previously detected wide and bizarre QRS complexes were no longer seen. Echocardiography was unchanged from the previous examination except that the left atrial to aortic diameter ratio on this occasion had a mean value of 1.65. The cat’s medication was not altered.
The cat was reassessed on two further occasions at three monthly intervals. No clinical signs were observed by the owner and repeat biochemistry and cTnI measurements were within normal limits. Electrocardiography identified AF with very occasional wide and bizarre QRS complexes. Echocardiography was repeated on both occasions and no significant changes were noted apart from a reduction in the size of the left atrium. The mean value for the left atrial to aortic diameter ratio was 1.59 on the fourth visit and 1.34 on the fifth visit. These findings indicate that not only was no structural disease identified but also that the left atrial size appeared to be decreasing to the normal range.
Figure 3 Pulsed wave Doppler tissue imaging of the interventricular septum at the level of the mitral annulus
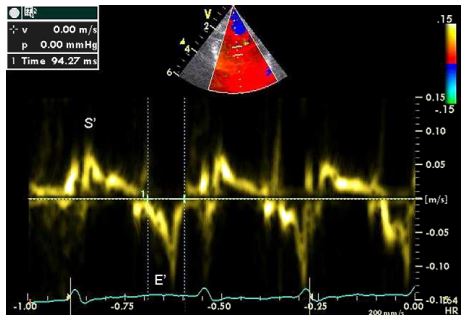
S’ is the positive systolic wave associated with ventricular contraction and E’ is the negative diastolic wave associated with ventricular relaxation. Note that the A’ wave linked to atrial contraction is not seen. At a heart rate of 164 bpm waveform summation is unlikely.
Pulsed wave tissue Doppler imaging of the interventricular septum and left ventricular free wall at the level of the mitral annulus was performed on the fifth examination and the A’ wave associated with atrial contraction was not evident6 (Fig. 3).
A final examination was performed 18 months after the initial referral and 1 year after the fifth visit. The owner had not noticed any clinical sign and no changes to the treatment regime have been made. The cat remained in AF and an enlarged left atrium (left atrial to aortic diameter ratio of 1.62) was seen on echocardiography. The rest of the examination was as previously described.
Discussion
Since the interesting aspect of this case was the presence of atrial fibrillation (AF) in a cat without enlarged atria at initial presentation, care was taken to ensure that the ECG diagnosis was correct and that echocardiographic measurements of the left atrium were carefully performed and an average of three readings was obtained. Furthermore the right atrium appeared to be smaller than the left atrium from the right parasternal short and long axis views but was not measured on the first visit.
On auscultation the rhythm sounded irregular and simultaneous display of the six leads of the ECG was consistent with a diagnosis of AF (variable R to R interval and no visible P waves). However, because of the absence of visible P waves alternative interpretations of the ECG although unlikely are possible including intermittent supraventricular ectopy conducted with aberrancy. Another problem in cats is that the atrial activation is so rapid and of such low voltage that it can be difficult to distinguish fibrillatory waves from baseline interference.1 Indeed 50 Hz alternating current interference is present on the ECG taken from this cat (Fig. 1).
The echocardiographic observations would make a diagnosis of atrial fibrillation (AF) more probable since on M-mode the A point of mitral valve septal leaflet movement and on conventional Doppler imaging the A wave of mitral inflow were absent at heart rates of below 170 bpm. On the last examination tissue Doppler imaging of the interventricular septum and left ventricular free wall at the level of the mitral annulus was performed and again the A’ wave was absent at a heart rate of 164 bpm (Fig. 3). These observations were made at heart rates where summation of the relevant waveforms is unlikely suggesting that their absence was a result of impaired atrial contraction. In summary the irregular nature of the rhythm on auscultation, the variability of the R to R intervals on electrocardiography and the finding on echocardiography would make atrial fibrillation the most likely diagnosis.
Conventionally atrial fibrillation has been considered an end- stage event in cats with myocardial disease resulting in marked atrial enlargement.3 However, these conclusions have been based on a limited number of cases published several years ago when the prevalence of different forms of feline cardiomyopathy was different from now.7 Recently a retrospective study of 50 cats with atrial fibrillation (AF) suggests that this arrhythmia may be associated with a wider cohort of feline patients than was previously recognized.2 In this study it was found that the majority of cats were male (82%) and elderly (10.2 + 3.7 years) at the time of diagnosis of AF. They also found that in a substantial number of cats (24%) atrial fibrillation (AF) was diagnosed incidentally and these cats were presented for veterinary attention for reasons not related to cardiovascular disease. The cat in this present report was a 10-year-old male with an arrhythmia detected incidentally at a routine pre-vaccination examination and has never shown signs of cardiovascular compromise. The cat described here was consistently tachycardic with a heart rate between 165 and 200 bpm prior to the administration of atenolol, which was similar to the results of Cote et al. study.2 In 39/50 cats in this retrospective study2 where the left atrial to aortic diameter ratio was calculated, 34 cats had convincing left atrial enlargement with a left atrial to aortic diameter ratio of > 1.7. In the remaining 5 cats the left atrial to aortic diameter ratio was considered equivocal ranging between 1.3 and 1.7.
In this present report the mean ratio was 1.26 at the time of diagnosis and was therefore considered within normal limits by our own criteria and those of Cote et al.2 The ratio increased to a maximum of 1.84 at the first recheck, decreased to 1.34 at the fifth examination and was 1.62 at the last visit. These results would suggest that it is not an absolute requirement to have an enlarged left atrium to act as the substrate for the initiation and maintenance of atrial fibrillation (AF) in this species. It is appreciated that care needs to be taken when assessing left atrial enlargement using one imaging plane especially when the use of the left atrium to aortic ratio can be affected by an increase in diameter of the aorta in the older cat. For this reason the left atrium was also assessed from the right parasternal long axis view and measured 20 mm from the mitral valve to the apex and 16 mm from the free wall to the interatrial septum which was considered within normal limits. A reference range of atrial width of 7—17 mm based on M-mode analysis in normal cats has been published.8 Subjectively at all echocardiographic examinations right atrium appeared to be within normal limits and on the second visit measured 20 mm by 14 mm.
Lone atrial fibrillation (AF) defined as the presence of the arrhythmia in the absence of detectable cardiac disease has been described in the dog primarily affecting the giant breeds such as the Great Dane and Irish wolfhound.9—11 In the majority of cases, giant breed dogs with AF that did not die of some other cause eventually showed convincing signs of dilated cardiomyopathy.11 Lone or paroxysmal AF is frequently recognized in humans and horses and in people is often treated using radiofrequency abla- tion.12—14 Indeed the ability to cardiovert atrial fibrillation (AF) back to a sinus rhythm by this method has resulted in the identification of ectopic foci (rotors) that originate from atrial musculature that extends into the pulmonary veins and from the posterior left atrial wall. Under correct electrophysiological conditions these foci may induce AF.15—17 Little is known about mechanisms of AF in the cat but Lone AF has not been described in this species to the author’s knowledge. In Cote et al. study cardiac structural abnormalities were identified in all 50 cats with AF and consisted of restrictive/unclassified cardiomyopathy in 19, left ventricular hypertrophy in 18, dilated cardiomyopathy in 6, and a variety of other cardiac conditions in the remainder.2 In this present report it is difficult to accurately define what form of underlying cardiac disease was present and it could be argued especially in the absence of significant echocardiographic changes or raised blood cTnI that there is insufficient evidence to conclude that the cat had significant intrinsic cardiac disease.17,18 The term unclassified cardiomyopathy is used when the disease profile does not fall into the criteria defined for the other forms of acquired myocardial disease.7 However, in the absence of a histopathological diagnosis the use of this term may not be correct in this case. At the final examination the left atrium had again enlarged and whether this was due to an underlying myocardial disease or as a consequence of the chronic arrhythmia remains uncertain. Particular systemic diseases have been associated with arrhythmias in the cat most noticeably hyperthyroidism.19 Appropriate tests were performed in this case to detect non-cardiac disease using blood tests and diagnostic imaging and none were found.
It is unlikely that the changes in size of the left atrium on different examination dates were due to operator’s error since the same person performed the echocardiogram on each occasion and when the atrium measured big it also subjectively appeared enlarged. This variation in atrial size was probably due to haemodynamic volume and pressure changes, which may have been induced by the medication.
Initially treatment was directed towards reducing the ventricular response rate to the atrial fibrillation using the b-blocker atenolol since the cat appeared chronically tachycardic. At the second visit because of the enlarged left atrium and potential increased risk for congestive heart failure an ACE inhibitor was introduced and the owner was given a supply of furosemide to use in an emergency situation. ACE inhibitors have been shown to reduce the degree of fibrosis acting as a substrate for AF in experimental canine models of heart failure and it has been suggested that they may be a suitable additional therapy for atrial fibrillation (AF) .20,21 Fibrosis has been identified in cats with enlarged left atria and AF and this fact would support the use of benazepril in this cat.22 It is difficult to give an accurate prognosis in this case especially since a definitive diagnosis of structural heart disease was not made. Cote et al. reported that 8/24 cats lived for more than 1 year after the diagnosis of AF was made and also showed that the median survival time for cats with overt clinical manifestations of illness at the time that atrial fibrillation (AF) was identified was not significantly different from the median survival time for cats in which AF was an incidental finding.2
This case illustrates an unusual presentation of atrial fibrillation (AF) in a cat because of the absence of left atrial enlargement or identifiable heart disease. Traditionally it has been suggested that the atrium must be of a sufficient size in order to sustain AF. The notion that atrial enlargement may predispose to the development of AF was based on the multiple wavelet hypothesis of AF which requires a certain atrial mass to be present to accommodate a critical number of wavelets which are required for self perpetuating fibrillation.23,24 Furthermore the greater the number of wavelets present in the atria at the same time the smaller the probability that they terminate simultaneously and therefore the more stable the atrial fibrillation (AF) becomes.25 Based on this concept a larger atrium should accommodate more wavelets at any one time than a smaller atrium.26 It is difficult to reconcile the presence of AF in this cat with the multiple wavelet theory in view of the small atrial size. Since the cat has not been used as an experimental model for AF little is known about the mechanism of this arrhythmia in this species. Interestingly it is possible to induce AF under experimental conditions in animals with very small atria, specifically in genetically altered mice. Different murine models used to study the genetic basis of atrial fibrillation (AF) have been described including transgenic mice over-expressing TGF-b 1 (resulting in atrial fibrosis)27 and knockout mice in which the potassium channel /KACh vital for the parasympathetic regulation of heart rate was deleted.28 There is of course no evidence to suggest that such genetic alterations caused the arrhythmia in the cat reported here.
References
- Gelzer ARM, Kraus MS. Management of atrial fibrillation. Vet Clin North Am Small Anim Pract 2004;34:1127-44.
- Cote E, Harpster NK, Laste NJ, et al. Atrial fibrillation in cats: 50 cases (1979-2002). J Am Vet Med Assoc 2004; 225:256-60.
- Fox PR. Diagnosis and management of feline arrhythmias. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. Principles and clinical practice. 2nd ed. Philadelphia: WB Saunders; 1999. p. 386-99.
- Syme HM, Barber PJ, Markwell PJ, Elliot J. Prevalence of systolic hypertension in cats with chronic renal failure. J Am Vet Med Assoc 2002;220:1799-804.
- Kienle RD. Echocardiography. In: Kittleson MD, Kienle RD, editors. Small animal cardiovascular medicine. St Louis: Mosby; 1998. p. 95-117.
- Gavaghan BJ, Kittleson MD, Fisher KJ, et al. Quantification of left ventricular diastolic wall motion by Doppler tissue imaging in healthy cats and cats with cardiomyopathy. Am J Vet Res 1999;60:1478-86.
- Fox PR. Feline cardiomyopathies. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. Principles and clinical practice. 2nd ed. Philadelphia: WB Saunders; 1999. p. 621-78.
- Sisson DD, Knight DH, Helinski C, et al. Plasma taurine concentrations and M-mode echocardiographic measures in healthy cats and cats with dilated cardiomyopathy. J Vet Intern Med 1991;5:232-8.
- Moise NS. Diagnosis and management of canine arrhythmias. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. Principles and clinical practice. 2nd ed. Philadelphia: WB Saunders; 1999. p. 331-85.
- Kittleson MD. Diagnosis and treatment of arrhythmias. In: Kittleson MD, Kienle RD, editors. Small animal cardiovascular medicine. St Louis: Mosby; 1998. p. 449-94.
- Brownlie SE, Cobb MA. Observations on the development of congestive heart failure in Irish wolfhounds with dilated cardiomyopathy. J Small Anim Pract 1999;40:371-7.
- Van Loon G, Duytschaever M, Tavernier R, et al. An equine model of atrial fibrillation: methodology. Vet J 2002; 164: 142-50.
- Jais P, Haissaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95:572-6.
- Haissaguerre M, Shah DC, Jais P, et al. Mapping guided ablation of pulmonary veins to cure atrial fibrillation. Am J Cardiol 2000;86:K9-19.
- Todd DM, Skanes AC, Guiraudon G, et al. Role of the posterior left atrium and pulmonary veins in human lone atrial fibrillation. Circulation 2003;108:3108-14.
- Jalife J. Rotors and spiral waves in atrial fibrillation. J Car- diovasc Electrophysiol 2003;14:776-80.
- Connolly DJ, Cannata J, Boswood A, et al. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003;5:209-16.
- Herndon WE, Kittleson MD, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002;16:558-64.
- Fox PR, Broussard JD, Peterson ME. Hyperthyroidism and other high outputstates. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. Principles and clinical practice. 2nd ed. Philadelphia: WB Saunders; 1999. p. 781-93.
- Li D, Shinagawa K, Li P, et al. Effects of angiotensin converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 2001; 104: 2608-14.
- Shi Y, Danshi L, Jean-Claude T, et al. Enalapril effects on atrial remodelling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res 2002;54: 456-61.
- Boyden PA, Tilley LP, Albala A, et al. Mechanisms for atrial arrhythmias associated with cardiomyopathy: a study of feline hearts with primary myocardial disease. Circulation 1984;69:1036-47.
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200-20.
- Allessie MA, Lammer WJ, Bonke FI, et al. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology and arrhythmias. Orlando: Grune and Stratton; 1985. p. 265-75.
- Allessie MA, Konings K, Kirchof CJHJ, et al. Electrophysiological mechanisms of perpetuation of atrial fibrillation. Am J Cardiol 1996;77:10A.
- Guglielmini C, Chetboul V, Pouchelon JL, et al. Influence of left atrial enlargement and body weight on the development of atrial fibrillation: retrospective study on 205 dogs. Vet J 2000;160:235-41.
- Verheule S, Sato T, Everett T, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res 2004;94:1458-65.
- Kovoor P, Wickman K, Maguire CT, et al. Evaluation of the role of /KACh in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol 2001;37:2136-43.
^Наверх









