Echocardiographic identification of atrial-related structures and vessels in horses validated by computed tomography of casted hearts - Equine Vet J. 2018

Author information
Vandecasteele T., Cornillie P., van Steenkiste G., Vandevelde K., Gielen I., Vanderperren K., van Loon G.Echocardiographic identification of atrial-related structures and vessels in horses validated by computed tomography of casted hearts. Equine Vet J. 2018 May 28. doi: 10.1111/evj.12969. [Epub ahead of print]
Abstract
BACKGROUND: Echocardiography is the imaging technique of choice for the equine heart. Nevertheless, knowledge about ulrasonographic identification of dorsally located structures and vessels, related to the atria, in horses is scarce.
OBJECTIVES: To describe the echocardiographic approach and the identification of structures and vessels in relation to the atria in healthy horses.
METHODS: CT images from two equine hearts, casted with self-expanding foam, were segmented and used to identify atrial-related structures and vessels. These images were compared with standard and non-standard ultrasound images from ten healthy horses obtained from a left and right parasternal view optimised to visualise the dorsal cardiac area.
RESULTS: On new standard ultrasound views, specific atrial anatomical landmarks such as vena cava, pulmonary arteries, intervenous tubercle and oval fossa were identified in all horses. In addition, ultrasound views were defined to visualise the brachiocephalic trunk, pulmonary veins and their ostia. The 3D segmented CT images from casted hearts were used to reconstruct slices that corresponded with the echocardiographic images and allowed correct identification of specific structures.
MAIN LIMITATIONS: Ultrasound exams and casts were from different animals. A small number of casts and horses were used therefore anatomical variation or individual differences in identifying structures on ultrasound could not be assessed.
CONCLUSIONS: Important cardiac structures and vessels, even the different pulmonary veins, could be identified on standard and non-standard ultrasound images in adult horses. This knowledge is important to guide and develop interventional cardiology and might be useful for diagnostic and therapeutic purposes. This article is protected by copyright. All rights reserved. This article is protected by copyright. All rights reserved.
KEYWORDS:
cardiac; horse; pulmonary veins; ultrasound
Introduction
While fluoroscopy, MRI and CT are widely used in human cardiology [1 -3], these techniques have limited value (fluoroscopy) or cannot be applied in adult horses (MRI, CT and in horses, echocardiography is the principal cardiac imaging tool. Currently, for routine cardiac assessment, the echocardiographic exam is performed in standard, predefined long and short axis image planes from the left and right thorax that allow identification and measurements of common structures such as atria, ventricles, myocardial walls, valves and the origin of aorta (Ao) and pulmonary artery (PA) [ 4 ].
While these rather ventrally located structures have been described in detail, there is limited information regarding more dorsally located structures such as dorsal atrial walls, cranial (CrVC) and caudal vena cava (CaVC), brachiocephali c trunk (BT) and pulmonary veins (PV) because they are partially covered by air -filled lungs, and therefore more difficult to visualise [ 5 - 7 ]. Recently, based upon a topographical study, the ultrasonographic identification of PV in horses was described [ 8 ] . Accepted Article This article is protected by copyright. All rights reserved. Invasive cardiac procedures are of increasing interest in equine cardiology .
Navigation of equipment through vessels and cardiac chambers relies on good ultrasonographic anatomical landmarks. More and more centres offer transvenous electrical cardioversion which requires accurate positioning of catheters in right atrium (RA) and left pulmonary artery (lPA) [9 - 1 4]. Pacemaker lead implantation, cardiac biopsy procedures and occluder implantation are performed with ultrasound guidance [15 -18]. Equine electrophysiological studies are performed both for research and for diagnostic purposes [19 -21]. Recently, the first successful 3D electro -anatomical cardiac mapping has been reported in adult horses [22 ]. Ultrasound guided electro -anatomical mapping from within the RA, left atrium (LA) and PV would be of great interest in horses treated for atrial fibrillation to assess PV ectopy [23], and could lead to development of new treatment strategies. The importance of the PV in atrial fibrillation pathophysiology has been well established in humans [24] but information is currently lacking in horses. Visualisation of the PV would also be of interest to assess PV size and flow. The aim of this study was to identify left and right atrial -related anatomical landmarks and blood vessels on ultrasound .
In order to do so , CT images from casted equine hearts were segmented, 3D reconstructed and subsequently used for comparison with ultrasound images to allow correct identification of the different structures. Materials and Methods Cardiopulmonary sets of four adult horses (2 Warmblood geldings, one Thoroughbred mare and one Quarter horse stallion; body weight 530 -600 kg; age 2.5 -6 years), euthanised for non -cardiovascular reasons, were removed immediately after death with preservation of the proximal part of CrVC, CaVC and Ao .
Via an incision in the left and right auricle, after Accepted Article This article is protected by copyright. All rights reserved. removal of blood clots, one -component self -expanding polyurethane construction foam a was injected through a tube, placed into the right (RV) and left ventricle (LV). Whilst the foam was injected, the tube was slowly retracted towards the atria. Two hours after foam injection the cardiopulmonary set was subjectively evaluated to represent a close to normal expansion of all cardiac chambers and vessels (Fig 1). Of the two best casted hearts, distal parts of the lungs were removed, leaving the PV intact, and computed tomographic (CT) scans were made with a four -slice helical CT device at 120 kV and 140 mA (CT scanner, LightSpeed, Qx/I b ) .
The obtained CT DICOM image s from both hearts were imported in dedicated computer software (Amira 6.1 3D software c ) and 3D -reconstructed. Subsequently, within the software program , cardiac chambers and associated vessels were segmented with specific colour codes (see Fig 2 ) to allow quick and easy orientation of the 3D reconstruction. In 10 horses (9 Warmblood s and one Thoroughbred) echocardiograph y (GE Vivid 7 Dimension with 3S phased array transducer at 1.7/3.4 MHz d ) was performed from a left and right parasternal approach focussing on the dorsal cardiac region to identify left and right atrial -related anatomical structures and blood vessels. Different non -standard views were used to identify important structures and vessels. Most of these views had been used by our research group during previou s invasive cardiac procedures (G. van Loon, unpublished data). For each echocardiographic view , the transducer handling was accurately described by defining probe position and rotation (Table 1 ) . Probe position refers to the spot where the transducer was placed on horse’s thorax . Probe rotation describes how many degrees the probe was rotated clockwise (e.g. +90°) or counter clockwise (e.g. -90°) in regard to the neutral position (0°, index mark of the probe dorsally ) , which was close to the long axis view of the heart.
Finally, probe angulation was reported following the standardised nomenclature used for radiographic projections [25], approved by the American College of Veterinary Radiology. This nomenclature system describes the direction of the beam from the point where it enters the body to the point where it would exit the body, separated by a hyphen. Standard abbreviations, such as left (Le), right (Rt), cranial (Cr), caudal (Ca), dorsal (D) and ventral (V), are used and may be combined. A horizontal probe position, approximately perpendicular to the chest of the standing horse, is regarded as a standard, non -oblique view. For oblique views, angles of obliquity can be inserted in addition to the term ‘oblique’ (O). Angles were subjectively assessed. For example, a right parasternal four - chamber view requires the probe to point about 10° caudally compared to the standard probe direction and would be described as a right cranial to left caudal oblique view, or more precisely a Rt10Cr –LeCaO view.
For all images, the probe index mark was displayed right on screen.
Results
Most important structures and vessels related to the atria were identified on CT and ultrasound images in all horses . Below the ultrasound images are explained, using the CT image as reference and to identify different structures. Table 1 summarises most important findings. Right parasternal view From the standard four -chamber view (Rt10Cr -LeCaO) with slightly more cranial and dorsal angulation (Rt 5Cr10V -LeCa DO), ostium III could be easily identified by its pulmonary veins which travel from right to left and which are found adjacent to the more ventrally located RA and the more dorsally located right pulmonary artery (rPA) . Close to ostium III, the oval Accepted Article This article is protected by copyright. All rights reserved. fossa (OF) was visible as a small, thinned area in the interatrial septum (Supplementary Item 1) or, depending on the ultrasonographic view, as a round anechoic structure in the atrial septum. The limbus of the oval fossa, which is the prominent craniodorsal margin, could also be identified .
From the same view, ostium IV was visible at the left dorsal wall of the LA ( Fig 2A and B). With slightly more caudal angulation (Rt20Cr10V -LeCa DO) (Supplementary Item 1), ostium III and IV , and the right pulmonary artery (rPA ) disappeared while the opening of ostium II in the LA could be identified. This view also showed part of the debouchment of the CaVC, especially when angulating even more caudally (Rt30Cr10V - LeCa DO). On a short axis view at the aortic valve level (Rt 30V -Le DO and -60° rotation), displaying CaVC, RA, RV, LA and aortic valves, PV ostium II was visuali sed at the caudodorsal border of the LA ( Fig 2C and D). Pointing more dorsally (Rt 60V -LeDO ) and with -45° rotation showed ostium III, adjacent to the RA, and ostium IV debouching at the left dorsal wall of the LA. From the four -chamber view, the ultrasound beam was rotated clockwise to + 80° and angled dorsally and slightly more cranially (Rt45 V -LeDO) until the intervenous tubercle (IT) was identified as a triangular myocardial structure originating from the right atrial wall (Fig 3A and B). This view showed the debouchment of the CrVC and CaVC into the RA. Deep to the RA, the bifurcation between Ao and BT was visible. Caudal to the IT, the thin wall of the OF with its limbus was seen, adjacent to the debouchment of ostium III. Craniomedial to ostium III, adjacent to the Ao, the rPA was found while deep to ostium III, ostium II was visible (Fig 3A and B).
When angulating more cranially (Rt10Ca45V -LeCrDO), the most caudal part of the CrVC could be identified. Placing the probe in the 3rd intercostal space with dorsal angulation (Rt60V -LeDO) and +80° rotation also allowed visualisation of the terminal Accepted Article This article is protected by copyright. All rights reserved. crest (TC) and right atrial appendage (RAA) in 3 horses ( Fig 3C and D). For this view, the horse’s right foot had to be moved forward, and substantial pressure with the probe against the triceps muscle was needed. In only one horse, the vena azygos could be seen by maximal dorsal angulation (Rt70V -LeDO) . Left parasternal view From a left parasternal long axis two -chamber view (Le -Rt, rotation 0° ), LA, LV and mitral valve were visualised. With slight cranial angulation (Le10Ca -RtCr O ), a longitudinal section through ostium III was identified on the right side of the heart, close to the mitral valve annulus, between the CaVC and rPA ( Fig 4A and B). By positioning and/or pointing the probe slightly more dorsally (Le10Ca10V -RtCrDO), the LA was centred on the image and the dorsal aspects of the LA became better visible. Subsequently, slight caudal angulation (Le 10Cr10 V - RtCa DO) displayed ostium IV and a longitudinal section through ostium II (Fig 4C and D; Supplementary Item 2). Compared to ostium III, ostium II was located at a greater distance from the mitral valve annulus. Ostium IV originated more dorsally and to the left of ostium II (Fig 4C and D). From the third intercostal space with cranial and slight dorsal angulation (Le45Ca10VRtCrDO) and no rotation (0°), first, the RA, RV, pulmonary valve and the base of the pulmonary trun k (PT) were visualised.
For this image, the horse’s left foot was moved forward and substantial pressure against the triceps muscle was needed. Subsequently, the course of the PT was followed by gradually rotating the probe clockwise and simultaneously angling it dorsally and caudally. With the probe rotated at about +80° and with dorsal and slight cranial angulation (Le15Ca60V -RtCrDO), the bifurcation of the PT into lPA and rPA was Accepted Article This article is protected by copyright. All rights reserved. visible (Fig 5A and B). On this image , the aorta was centrally located with the PT curved around it caudolaterally, while the CrVC and RAA located deep to the Ao . From this cranial PT bifurcation image, the probe was swapped to the 4th intercostal space , slightly more caudally angulated (Le60V -RtDO) and slightly counter clockwise rotated to +60°. On this image, the LA was found lateral to the longitudinally sectioned PT and its bifurcation in rPA and lPA (Fig 6 A and B), while the transverse section of the Ao was seen craniomedial to the PT. During the cardiac cycle the PT often moved in and out of the image. Adjacent to the PT bifurcation, near the lPA, a transverse section through ostium IV could be identified, entering into the LA. The rPA and lPA did not always appear simultaneously and rPA visualisation often required minimal beam adjustment. From this caudal PT bifurcation image, dorsal probe angulation was slightly reduced and slightly angled caudally (Le10Cr45V -RtCaDO) and rotated towards +80° until the PT disappeared and the LA was in the centre of the image. From this view pulmonary veins could be seen entering the LA. Minimal change in dorsal to ventral angulation and rotation, allowed to visualise (simultaneously or separately) a longitudinal section through ostium II and ostium III (Fig 6C and D ), whereby ostium II was found caudal to ostium III , and the rPA in between both vessels . A similar image could be obtained from the 5th intercostal space with slight cranial angulation (Le10Ca45V -RtCrDO) and the same rotation ( +80°). When the beam was further angulated ventrally, the mitral valve became visible.
Discussion
In human medicine, trans oesophageal and transthoracic (apical) views allow fairly good visualisation of the dorsal atria and pulmonary veins [26,27]. The latter have been extensively studied because of their importance in the pathophysiology and therapy of atrial Accepted Article This article is protected by copyright. All rights reserved. fibrillation, however, visualisation, measurements and therapeutic procedure guidance are all almost exclusively based on CT or MR images. New standard views were created to visuali se specific structures and anatomical landmarks and many views have become part of routine cardiac interventions in our research group. Although for small animals the Echocardiography Committee of The Specialty of Cardiology has recommended a short axis image display orientation with the cranial part of the heart right on screen [28], we deliberately choose to have exceptions to this rule. First, in the standing horse, many equine cardiologist s prefer a different probe rotation to obtain short axis images, whereby the caudal heart is displayed right on screen [29]. More importantly, our main goal was to allow for guidance of catheters or devices during their transvenous insertion for cardiac interventions, without having to rotate the probe almost 180°.
For a right parasternal view this means starting with a probe rotation of +90° to visuali se a device entering from CrVC into RA and subsequently following it towards the RV by counter clockwise probe rotation towards the four -chamber view at 0°. From this view, it was relatively easy to continue ultrasound guidance towards the pulmonary artery on the right ventricular inflow -outflow image. For ultrasound guidance from the left parasternal view, the device was visuali sed from a cranial view of the pulmonary valve and PT (0°). By gradually pointing more dorsally, at the same time rotating clockwise and then positioning it in the 4th intercostal space, an intracardiac device could be followed nicely along the PT towards the bifurcation. On that final image, the caudal part of the heart was positioned on the right of the screen. From this view, minimal ventral angulation displayed the LA with its pulmonary veins, again with the caudal aspect of the heart right on screen. Accepted Article This article is protected by copyright. All rights reserved.
In standard echocardiography, probe handling, especially angulation, is generally not described in much detail. The heart is easy to find on ultrasound and fine -tuning of probe angulation is mainly based upon the reproduction of a standard image on screen. We noticed that, even experienced equine cardiologists, initially did find some views challenging to acquire and interpret. Indeed, the cardiac window for these images is narrow, difficult to find and on the edge of the lung field while probe manipulation is very subtle. We therefore choose to provide more detail regarding probe manipulation based upon the standardi sed nomenclature used for radiographic projections [25], approved by the American College of Veterinary Radiology.
It is an easy to use system to describe oblique views in detail and the nomenclature is well know n amongst veterinarians and in veterinary literature. We assessed angles of obliquity subjectively. We did not make attempts to measure these angles more accurately because due to substantial individual variation, absolute angle values are not applicable for each individual horse. We rather wanted to provide guidance on how to obtain an image and how to manoeuvre from one image to another. Identifying specific landmarks such as IT, OF, limbus, vena cava, and the position and side branches of PV, PA and Ao has so far proven to be extremely helpful to achieve a well - controlled insertion of devices into the heart (G. van Loon , unpublished data). Visuali sation of OF and limbus could be very useful to develop a technique for ultrasound -guided transseptal puncture. With such a technique, access to the left heart without the need of retrograde arterial catheterisation could be obtained, which would allow pressure monitoring, electrophysiological studies, biopsy or device insertion . Accepted Article This article is protected by copyright. All rights reserved.
Visualisation and correct identification of the PV and their relation to other vessels was also one of the aims of our study. These vessels are of particular interest to study atrial tachyarrhythmia pathophysiology in horses and might become a target for future treatment strategy. In addition, as in small animal medicine, PV diameter or flow measurements could be useful to assess specific heart disease such as mitral regurgitation severity [30 -32] .
Except for the azygos vein and the PV ostium I, and in some horses the RAA and TC, most important atrial related structures could be identified in all horses. Due to individual variation and body condition, some views were more challenging to take than others and required some practicing. Imaging of the PV ostia, for example, was sometimes more difficult as these orifices are located at the border of the acoustic window. The visibility of ostium I depends on its location and shows anatomical variation in horses [8]. Equine cardiovascular imaging by radiography or MRI is very limited or impossible due to the size of the animal, making ultrasound the most important imaging modality. Our study provides new standard views for improved visualisation and 3D understanding of important anatomical landmarks and vessels in relation to the heart.
Indeed, in the future, additional approaches and views may be found helpful to visuali se specific regions. Thorough ultrasound knowledge of the entire heart will facilitate more accurate diagnostic examinations and is required to develop advanced invasive cardiac procedures in adult horses. Authors’ declaration of interests No competing interests have been declared. Accepted Article This article is protected by copyright. All rights reserved. Ethical animal research The current study (EC 2015/96) was performed following the guidelines of the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium. Owners gave informed consent for their animals’ inclusion in the study. Sources of funding Glenn Van Steenkiste received a Doctoral (PhD) grant Strategic Basic Research of FWO Flanders, Belgium. Acknowledgements The authors thank Bart De Pauw for the technical assistance and Dr Elke Van der Vekens for advice. Manufacturers’ addresses aHubo, Belgium. bGE Medical Systems, Milwaukee, Wisconsin, USA. c Thermo Fisher, Massachusetts, USA. dGE Healthcare, Diegem, Belgium. Accepted Article This article is protected by copyright. All rights reserved.
Table legend Table 1 : Probe angulation and rotation with corresponding visible structures on ultrasound from a right and left parasternal view.
Figure legends
Fig 1 : Casted heart after injection of polyurethane foam into the heart and major vessels. Panel A: the foam cast with the surrounding cardiac tissue still present was used to make the CT images. Panel B: foam cast without the surrounding cardiac tissue.
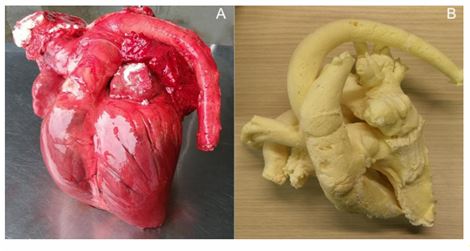
Fig 2 : Panels A and B: right parasternal long axis four -chamber view on ultrasound (A) and 3D - reconstructed CT image (B) visualising right atrium (RA), right ventricle (RV), left atrium (LA), left ventricle (LV), ostium III (III) and ostium IV (IV). From the 4th intercostal space, the ultrasound beam points slightly from right cranioventral to left caudodorsal (Rt5Cr10V -LeCaDO) with about 0° rotation. Panels C (ultrasound) and D (CT image): right parasternal short axis view at aortic valve level (Rt30V -LeDO, rotation -60°) displaying caudal vena cava (CaVC), right atrium (RA), right ventricle (RV), left atrium (LA), aortic valve (AV) and ostium II (II). Please see key for colour coding for the different chambers and associated vessels, used for segmentation of the CT images.
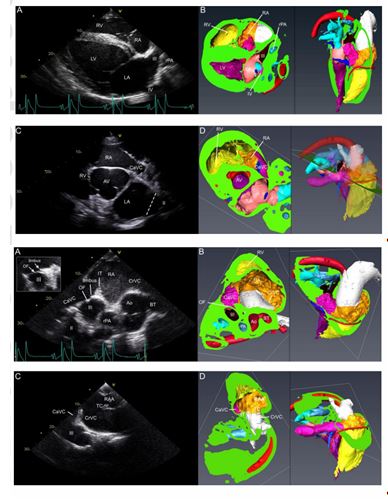
Fig 3 : Right parasternal short axis view from the 4th (A) and 3rd (C) intercostal space with corresponding CT images (B and D). Panel A: From the right 4th intercostal space, with +80° rotation and dorsal angulation (Rt45V -LeDO), the cranial (CrVC) and caudal vena cava (CaVC), separated by the intervenous tubercle (IT), are seen entering the right atrium (RA). Deep to the RA, the brachiocephalic trunk (BT), aorta (Ao), right pulmonary artery (rPA), ostium III, ostium II and oval fossa (OF) with limbus can be identified. In this horse, the limbus is more clearly visible at a different time point during the cardiac cycle (insert on the left). Panel C: from the 3rd intercostal space and with dorsal and cranial angulation (Rt60V -LeDO) and +80° rotation, the right atrial appendage (RAA) Accepted Article This article is protected by copyright. All rights reserved. and terminal crest (TC) can be seen, in addition to the cranial vena cava (CrVC), caudal vena cava (CaVC) , intervenous tubercle (IT) and ostium III (III) . Please see colour coding key in Figure 2.
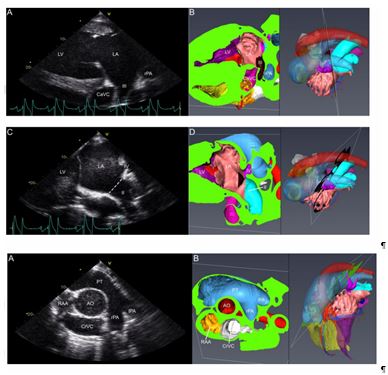
Fig 4 : Left parasternal long axis views with echocardiographic (A and C) and corresponding 3D - reconstructed (B and D) images. Panel A: 4th intercostal space with slight cranial angulation (Le10Ca - RtCr O) and no rotation (0°), visualising left ventricle (LV), left atrium (LA) and ostium III (III) flanked by caudal vena cava (CaVC) and right pulmonary artery (rPA). Panel C: compared to Fig 4A, the probe is now pointing slightly more dorsally and caudally (Le10Cr10V -RtCaDO); besides left ventricle (LV) and left atrium (LA), ostium II (II) and ostium IV (IV) are now visualised . The dotted line indicates the connection of ostium II with the LA . Please see colour coding key in Figure 2.
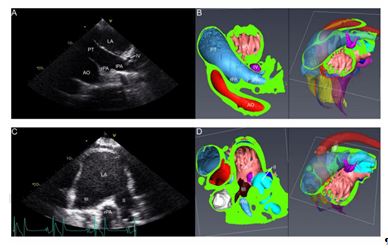
Fig 5 : Ultrasound (A) and corresponding CT image (B). Panel A: left parasternal oblique view from the 3rd intercostal space with +80° rotation and dorsal angulation (Le15Ca60V -RtCrDO), visualizing the bifurcation of the pulmonary trunk (PT) into left (lPA) and right pulmonary artery (rPA). The aorta (Ao) is centrally located while the cranial vena cava (CrVC) and the right atrial appendage (RAA) are deep to the Ao . Please see colour coding key in Figure 2.
Fig 6 : Left parasternal 4th intercostal echocardiographic (A&C) and corresponding CT (B&D) images from oblique views with dorsal angulation and +60° to +80° rotation. Panels A (Le60V -RtDO, rotation +60°) and B show the pulmonary trunk (PT) and the bifurcation into left (lPA) and right (rPA) pulmonary artery, caudolaterally flanked by left atrium (LA) and craniomedially by aorta (Ao). A cross section through ostium IV (IV) is found adjacent and lateral from the PT bifurcation. Panel C is taken with slightly less dorsal angulation (Le10Cr45V -RtCaDO) and a rotation of +80° and shows the left atrium (LA) with ostium III (III) and the more caudodorsally located ostium II (II). Between both ostia the rPA is found. Please see colour coding key in Figure 2. Accepted Article This article is protected by copyright. All rights reserved.
References
- Farré, J., Anderson, R.H, Cabrera, J.A., Sánchez -Quintana, D., Rubio, J.M., Benezet -Mazuecos, J., Del Castillo, S. and Macia, E. (2010) Cardiac anatomy for the interventional arrhythmologist: I. terminology and fluoroscopic projections. Pacing Clin . Electrophysiol . 33, 497 -507.
- Bluemke, D.A., Achenbach, S., Budoff, M., Gerber, T.C., Gersh, B., Hillis, L.D., Hundley, W.G., Manning, W.J., Printz, B.F., Stuber, M. and Woodard, P.K. (2008) Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation 118, 586 -606.
- Schuetz, G.M., Zacharopoulou, N.M., Schlattmann, P. and Dewey, M. (2010) Meta -analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann . Intern . Med . 152, 176 -177.
Feigenbaum, H. (1986) Echocardiography. 4th edn., Lea & Febiger, Philadelphia.
- Patteson, M. (1999) Two -dimensional and M -mode echocardiography. In: Cardiology of the Horse, Ed s: C.M. Marr. W.B. Saunders, London. pp 93 -116.
- Marr, C.M. and Patteson, M. (2010) Echocardiography. In: Cardiology of the Horse, 2nd edn., Ed: C.M. Marr and M. Bowen. Saunders Elsevier, Amsterdam, pp 3 -21.
- Palgrave, K. and Kidd, J.A. (2014) Introduction. In: Atlas of Equine Ultrasonography, 1st edn, Ed: J.A. Kidd, K.G. Lu and M.L. Frazer . John Wiley & Sons, New Jersey. pp 1 -22.
- Vandecasteele, T., van Loon, G., Vandevelde, K., De Pauw, B., Simoens, P. and Cornillie, P (2016) Topography and ultrasonographic identification of the equine pulmonary vein draining pattern. Vet . J . 210, 17 -23. Accepted Article This article is protected by copyright. All rights reserved.
- Alt, E., Ammer, R., Schmitt, C., Evans, F., Lehmann, G., Pasquantonio, J. and Schömig, A. (1997) A comparison of treatment of atrial fibrillation with low -energy intracardiac cardioversion and conventional external cardioversion. Eur . Heart J . 18, 1796 -1804.
- Levy, S., Lauribe, P., Dolla, E., Kou, W., Kadish, A., Calkins, H., Pagannelli, F., Moyal, C., Bremondy, M. and Schork, A. (1992) A randomized comparison of external and internal cardioversion of chronic atrial fibrillation. Circulation 86, 1415 -1420.
- McGurrin, M.K., Physick -Sheard, P.W. and Kenney , D.G. (2005) How to perform transvenous electrical cardioversion in horses with atrial fibrillation. J . Vet . Cardiol . 7, 109 -119.
- Schauvliege, S., van Loon, G., De Clercq, D., Devisscher, L., Deprez, P. and Gasthuys, F. (2009) Cardiovascular responses to transvenous electrical cardioversion of atrial fibrillation in anaesthetized horses. Vet . Anaesth . Analg . 36, 341 -351.
- De Clercq, D., van Loon, G., Schauvliege, S., Tavernier, R., Baert, K., Croubels, S., De Backer, P. and Deprez, P. (2008) Transvenous electrical cardioversion of atrial fibrillation in six horses using custom made cardioversion catheters. Vet . J . 177, 198 -204.
- van Loon, G., De Clercq, D., Tavernier, R., Amory, H. and Deprez, P. (2005) Transient complete atrioventricular block following transvenous electrical cardioversion of atrial fibrillation in a horse. Vet . J . 170, 124 -127.
- van Loon, G., Fonteyne, W., Rottiers, H., Tavernier, R. and Deprez, P. (2002) Implantation of a dual -chamber, rate -adaptive pacemaker in a horse with suspected sick sinus syndrome. Vet . Rec . 151, 541 -545.
- Decloedt, A., de Clercq, D., Ven, S., van der Vekens, N., Chiers, K. and van Loon, G. (2016) Right atrial and right ventricular ultrasound -guided biopsy technique in standing horses. Equine Vet . J . 48, 346 -351. Accepted Article This article is protected by copyright. All rights reserved.
- Javsicas, L.H., Giquère, S., Maisenbacher, H.W., Schmidt, M., Frederick, J.D., Conway, J.A. and Estrada, A.H. (2010) Percutaneous transcatheter closure of an aorta -cardiac fistula in a Thoroughbred stallion using an Amplatzer occluder device. J . Vet . Intern . Med . 24, 994 -998.
- van Loon, G., Fonteyne, W., Rottiers, H., Tavernier, R., Jordaens, L., D'Hont, L., Colpaert, R., De Clercq, T. and Deprez, R. (2001) Dual-chamber pacemaker implantation via the cephalic vein in healthy equids. J . Vet . Intern . Med . 15 , 564 -571 .
- Clercq, D., Decloedt, A., De Baere, S., Devreese, M., Van der Vekens, N., Ven, S., Croubels, S. and van Loon, G. (2016) Pharmacokinetics of intravenously and orally administered sotalol hydrochloride in horses and effects on surface electrocardiogram and left ventricular systolic function. Vet . J . 208, 60 -64.
- De Clercq, D., Decloedt, A., Sys, S.U., Verheyen, T., Van Der Vekens, N. and van Loon, G. (2014) Atrial fibrillation cycle length and atrial size in horses with and without recurrence of atrial fibrillation after electrical cardioversion . J . Vet . Intern . Med . 28 , 624 -629.
- Decloedt, A., De Clercq D., Van Der Vekens, N., Verheyen, T. and van Loon, G. (2014) Noninvasive determination of atrial fibrillation cycle length by atrial colour tissue Doppler imaging in horses. Equine Vet . J . 46 , 174 -179.
- van Loon, G., Boussy, T., Vera, L., De Clercq, D., Schauvliege, S., Vandecasteele, T., Decloedt, A., Van Steenkiste, G. and Van Langenhove, G. (2017) Electroanatomical cardiac mapping in an adult horse in sinus rhythm. J . Vet . Intern . Med . 31, 1572 -1604.
- Young, L.E. (2003). Equine athletes, the equine athlete’s heart and racing success. Exp . Physiol . 88, 659 -663. Accepted Article This article is protected by copyright. All rights reserved.
- Haïssaguerre, M., Jaïs, P., Shah, D.C., Takahashi, A., Hocini, M., Quiniou, G., Garrigue, S., Le Mouroux, A., Le Métayer, P. and Clémenty, J. (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N . Engl . J . Med . 339, 659 -666.
- Smallwood, J.E., Shively, M.J., Rendano, V.T. and Habel, R.E. (1985) A standardized nomenclature for radiographic projections used in veterinary medicine. Vet . Radiol . 26, 2 -9.
- Huang, X., Huang, Y., Huang, T., Huang, W. and Huang, Z. (2008) Individual pulmonary vein imaging by transthoracic echocardiography: an inadequate traditional interpretation. Eur . J . Echocardiogr . 9, 655 -660.
- Baer, J., Wyatt, M.M. and Kreisler, K.R. (2018) Utilizing transesophageal echocardiography for placement of pulmonary artery catheters. Echocardiogr . doi: 10.1111/echo.13812.
- Thomas, W.P., Gaber, C.E., Jacobs, G.J., Kaplan, P.M., Lombard, C.W., Moise, N.S. and Moses, B.L. (1993) Recommendations for standards in transthoracic two -dimensional echocardiography in the dog and cat. J . Vet . Intern . Med . 7, 247 -252.
- Long, K.J., Bonagura, J.D. and Darke, P.G. (1992) Standardised imaging technique for guided M - mode and Doppler echocardiography in the horse. Equine Vet . J . 24, 226 -235.
- Tabata, T., Thomas, J.D. and Klein, A.L. (2003) Pulmonary venous flow by Doppler echocardiography: Revisited 12 years later. JACC 41, 1243 -1250.
- Brewer, F.C., Moïse, N.S., Kornreich, B.G. and Bezuidenhout, A.J. (2012) Use of computed tomography and silicon endocasts to identify pulmonary veins with echocardiography. J . Vet . Cardiol . 14, 293 -300.
- Merveille, A.C., Bolen, G., Krafft, E., Roels, E., Gomart, S., Etienne, A.L., Clercx, C. and Mc Entee, K. (2015) Pulmonary Vein -to -Pulmonary Artery Ratio is an Echocardiographic Index of Congestive Heart Failure in Dogs with Degenerative Mitral Valve Disease. J . Vet . Intern . Med . 29, 1502 -1509.
Accepted Article This article is protected by copyright.
All rights reserved. Supporting information Supplementary Item 1: Video of right parasternal long axis images .
This article is protected by copyright. All rights reserved. Supporting information Supplementary Item 1: Video of right parasternal long axis images . Supplementary Item 2:
Video of left parasternal long axis images . Probe angulation, rotation Visible structures* Right parasternal view Rt5Cr10V -LeCaDO 0° RA, RV, LA, LV, rPA, III, IV, OF, limbus Rt20Cr10V -LeCaDO 0° RA, RV, LA, LV, II Rt30Cr10 V -LeCaDO 0° RA, RV, LA, LV, II, CaVC Rt30V -LeDO -60° RA, RV, LA, AV, II, CaVC Rt60V -LeDO -45° RA, RV, LA, A o, III, IV Rt45V -LeDO +80° RA, CrVC, CaVC, IT, OF, limbus, III, II, BT, Ao, rPA, Rt60V -LeDO +80° RAA, TC, CrVC, CaVC, IT, III Left parasternal view Le10Ca -RtCrO 0° LA, LV, III, RV, CaVC, rPA Le10Cr10V -RtCaDO 0° LA, LV, II, IV Le45Ca10V -RtCrDO 0° RA, RV, PT, pulmonary valve Le15Ca60V -RtCrDO +80° PT, lPA, rPA, Ao, CrVC, RAA Le60V -RtDO +60° PT, lPA, rPA, LA, Ao, IV Le10Cr45V -RtCaDO +80° LA, II, III, rPA * for abbreviations, see Table 1. (AV: aortic valve; BT: brachiocephalic trunk; IT: intervenous tubercle; OF: oval fossa; RAA: right atrial appendage; TC:
^Наверх









