Clinical and echocardiographic findings of pulmonary artery stenosis in seven cats

Author information
Schrope D.P., Kelch W.J. Clinical and echocardiographic findings of pulmonary artery stenosis in seven cats // J Vet Cardiol. 2007 Nov;9(2):83-9.
Abstract
OBJECTIVES: Describe the clinical, electrocardiographic (ECG), radiographic and echocardiographic findings in cats with isolated pulmonaryartery stenosis. Assess the usefulness of systolic and diastolic Doppler measurements at predicting stenosis severity. BACKGROUND: Pulmonary artery stenosis is an infrequent congenital cardiac defect in humans that has not been reported in cats. In humans, pulmonary artery stenosis is usually seen in conjunction with other cardiac defects and may lead to clinical signs if severe. ANIMALS, MATERIALS AND METHODS: Seven cats with pulmonary artery stenosis were retrospectively evaluated. Medical records, radiographs, ECGs, echocardiograms and angiocardiograms were reviewed. Severity of stenosis was assessed by two-dimensional and color Doppler echocardiographic evaluation and clinical findings. Peak systolic and diastolic gradients across the stenosis, and systolic and diastolic pressure decay half-times were graded using echocardiography. In addition, the duration of antegrade flow during diastole was subjectively assessed. Univariate analyses were performed to assess the best variable to predict stenosis severity. RESULTS: Concurrent congenital defects were not identified. Only cats with severe obstruction showed clinical signs including exertional dyspnea and lethargy. Diastolic Doppler measurements were superior to systolic measurements at predicting severity of stenosis. Antegrade flow throughout diastole and/or a diastolic pressure half-time of >100 ms indicated severe obstruction. The prognosis for pulmonary arterystenosis appears to be good regardless of severity. CONCLUSION: Among cats with pulmonary artery stenosis, clinical signs are uncommon and prognosis is good. Doppler assessment of diastolic flow appears to be superior to systolic flow at predicting severity.
Congenital stenosis of the main and/or branched pulmonary arteries has been identified in humans and is commonly identified with concurrent lesions such as ventricular septal defect, pulmonic stenosis, patent ductus arteriosus, or tetralogy of Fallot.1-3 Congenital obstruction of the right ventricular outflow tract is uncommon in cats with an incidence of about 2-3% of congenital cardiac defects.4,5 To the authors’ knowledge, stenosis of the main or branched pulmonary arteries has not been reported in a cat. Stenosis of the pulmonary artery is hemody- namically similar to coarctation of the aorta and was referred to as coarctation of the pulmonary artery in early human studies.1 As with other types of stenotic lesions, Doppler echocardiography has been used to assess the severity of aortic coarctation using the modified Bernoulli’s equation to estimate the systolic pressure gradient across the obstruction, however, this does not consistently reflect severity of the stenosis when compared to gradients measured at cardiac catheterization.6,7 Further studies of aortic coarctation in humans have identified variable degrees of diastolic flow across the stenosis. Both subjective and objective assessment of this diastolic flow has been found to be more accurate than systolic gradients at assessing severity of coarctation in many patients.6,8,9 In humans with pulmonary artery stenosis and in experimental studies of pulmonary artery banding in dogs, there are varying conclusions about how closely the Doppler systolic and cardiac catheterization-derived gradients correlated.10,11 In cats with pulmonary artery stenosis, it was hypothesized that diastolic Doppler evaluation would relate better to severity than systolic evaluation.
Animals, materials and methods
Cats with an echocardiographic diagnosis of pulmonary artery stenosis were retrospectively reviewed. The majority of these cats were identified through an animal shelter that receives animals from multiple sites along the east coast of the United States. All of the cats were initially evaluated because a murmur had been ausculted on physical exam. The P-wave and QRS complex amplitude and duration, and the PR interval were measured on three consecutive cardiac cycles and averaged on the electrocardiograms that were available for review. The mean QRS axis was also assessed. The vertebral heart scale (VHS) was measured on all lateral and ventrodorsal radiographs that were available for review.12 Complete echocardiograms were performed using standard echocardiographic views. Maximum pulmonary valve annulus diameter (PVD) and pulmonary artery stenosis diameter (PSD) were obtained from right or left parasternal short-axis two-dimensional (2D) images during systole. Pulmonary artery stenosis diameter was evaluated with the assistance of color-flow Doppler (CFD) to identify the vena contracta in all cases. In cats with severe disease the stenotic orifice was often so small that it was not easily identified without CFD. The ratio of stenosis diameter to pulmonary valve annulus diameter (PSD/PVD) was calculated. Maximum right atrial diameter (RAD) and left atrial diameter (LAD) were obtained from right parasternal long-axis 2D images during diastole. Measurements of the RAD were performed using a method similar to published methods of measuring the LAD in long-axis.13 The ratio of RAD to LAD was calculated and considered normal if <1.0.14 Right ventricular end-diastolic diameter (RVDd) and left ventricular end-diastolic diameter (LVDd) were obtained from M-mode tracings. The RVDd to LVDd ratio was generated and considered normal if <0.33.14 The remainder of the left heart measurements was made from standard 2D and M-mode echocardiographic views. All 2D and M- mode measurements were made on three consecutive cardiac cycles and averaged.
Peak systolic flow velocity through the stenosis was measured in all cats after normal pulmonary valve flows were confirmed and a velocity step-up was identified at the level of the stenosis. Estimation of the peak systolic pressure gradient (PaS) and peak diastolic pressure gradient (PaD) across the stenosis was calculated using the modified Bernoulli’s equation. Peak diastolic flow velocity was measured at the onset of diastole identified by the end of the T-wave on the electrocardiogram (Fig. 1).8 The systolic pressure decay half-time (Spht) was measured from the peak systolic flow to the onset of diastole (the end of the T-wave). The diastolic pressure decay half-time (Dpht) was measured from the onset of diastole to baseline or to the onset of systole if antegrade flow was present throughout diastole. All measurements were performed on three consecutive waveforms and averaged. The presence or absence of diastolic flow (DFA) was also subjectively assessed from the spectral Doppler tracing and graded as; (0+) no diastolic flow, (1+) flow through 50% or less of diastole, (2+) flow through >50% but <100% of diastole, and (3+) diastolic flow throughout diastole resulting in continuous flow across the stenosis (Fig. 1).
Angiocardiography was performed to better characterize the lesion in the two initial cases. The ratio of minimal stenosis diameter to PV annulus diameter was obtained and averaged from at least two cardiac cycles during systole.
Figure 1 Spectral Doppler findings in cats 3 (A) and 7 (B) and technique used to identify diastolic Doppler measurements
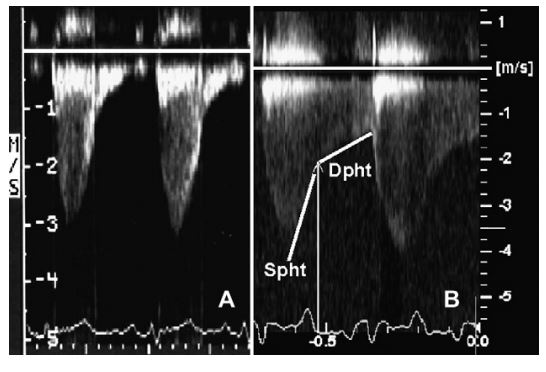
The onset of diastole was identified at the end of the T-wave and the peak diastolic gradient was measured at this point. The systolic pressure decay half-time (Spht) was measured from the peak systolic flow to the onset of diastole. The diastolic pressure decay half-time (Dpht) was measured from the onset of diastole to the end of diastolic flow.
For statistical analysis, severity of stenosis was assessed based on the echocardiographic PSD/PVD ratio. The diastolic and systolic Doppler data were compared to the PSD/PVD ratio. Univariate analyses were performed to assess the correlation with stenosis severity.c A p-value <0.05 was considered significant; r refers to the coefficient of linear correlation between two variables; r-squared for continuous variables refers to the coefficient of multiple determination which estimates the proportion of the response variation that can be explained by the continuous independent variable; and r-squared for an ordinal variable refers to the uncertainty coefficient which, analogous to the coefficient of multiple determination for continuous variables, also explains the proportion of the response variation explained by the ordinal independent variable.
Results
The signalment, clinical signs, and findings of cardiac auscultation are summarized in Table 1. None of the cats had evidence of jugular pulses, cyanosis, alterations in femoral pulse strength or quality, abnormalities in S1 or S2, or a gallop rhythm during a resting examination.
Six of the cats were asymptomatic at the time of presentation. One cat (diagnosed at 12 years of age) was described as less active than expected all of her life by one of two owners. One cat, that had initially been asymptomatic, developed moderate exertional dyspnea soon after diagnosis. An arterial blood gas was performed after dyspnea was induced by exercise. The arterial blood gas was assessed as a mixed metabolic and respiratory acidosis with hypoxia (pH 7.1, pCO2 45.6, pO2 84.0, HCO3 14.0, BE—15, sO2 90%). The calculated alveolar to arterial gradient was nine. The findings were consistent with hypoperfusion from pulmonary artery stenosis. The clinical signs in cat 7 did not progress over time, and no treatment was initiated.
Three cats had an ECG available for review. No abnormalities were identified in two of the cats (#2 and 6). The ECG of cat 5 revealed deep S-waves in leads I, II, III, aVF, a right mean electrical axis shift (170°—180°), and widening of the QRS complex (50 ms) suggestive of right ventricular enlargement or a partial right bundle branch block. Five cats (#3—7) had chest radiographs available for review. All five cats had normal VHS on lateral and ventrodorsal views and one cat (#7) had subjective evidence of right ventricular enlargement and a small pulmonary artery bulge.
Echocardiography was performed in all seven cats and identified pulmonary artery stenosis with variable degrees of main pulmonary artery dilation proximal to the stenotic lesion (Figs. 2 and 3). Color-flow Doppler confirmed turbulence during systole at the suspected stenosis in all cats and diastolic flow at the level of the stenosis in cats 6 and 7. No cat showed evidence of pulmonary valve disease or left heart abnormalities. A summary of the 2D echocardiographic findings of the right heart and spectral Doppler data is presented in Table 2. Visualization of the pulmonary artery bifurcation and branched pulmonary arteries from the right parasternal short-axis view was accomplished in the majority of cats. In cat 6 the branched pulmonary arteries could only be visualized from a left parasternal short-axis view. In cats 4 and 7 the branched pulmonary arteries could not be visualized well from the right or left side with 2D. The CFD in both cats, though, did show flow convergence and turbulence at the bifurcation with splitting of the color-flow pattern into the origins of the branched pulmonary arteries.
Table 1 Signalment, initial auscultation findings, and clinical signs in seven cats with pulmonary artery stenosis
Angiocardiography confirmed in cats 4 and 7 an isolated lesion of the distal main pulmonary artery just proximal to the bifurcation without involvement of the branched or peripheral segmental pulmonary artery (Fig. 4). The average ratio of PSD/PVD on the angiocardiograms was 0.50 in cat 4 and 0.32 in cat 7.
Based on the univariate analyses, the Dpht (p = 0.002; r = -0.94; r2 = 0.88) was most closely associated with stenosis severity. The DFA (p = 0.025; r2 = 0.94), PaD (p = 0.003; r =-0.92; r2 = 0.85) and PaS (p = 0.009; r =-0.88; r2 = 0.78) were also significantly associated with stenosis severity. Cats 6 and 7 had evidence of right atrial and ventricular dilation as well as clinical signs. It was felt that these two cats had severe disease. Both cats with severe stenosis had a DFA of 3+ diastolic flow and Dpht >100 ms (Table 2).
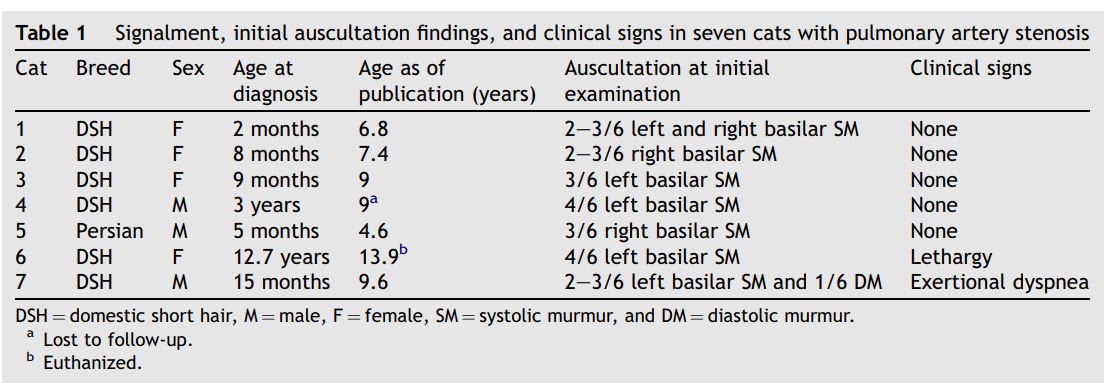
Figure 2 Two-dimensional (A) and color-flow Doppler (B) images of cat 1 with mild pulmonary artery stenosis
Images were obtained during systole. Note the mild narrowing of the pulmonary artery just proximal to the bifurcation (A) and associated flow convergence using color-flow Doppler (B). (Ao = aorta, MPa = main pulmonary artery, RPa = right-branched pulmonary artery, LPa = left-branched pulmonary artery).
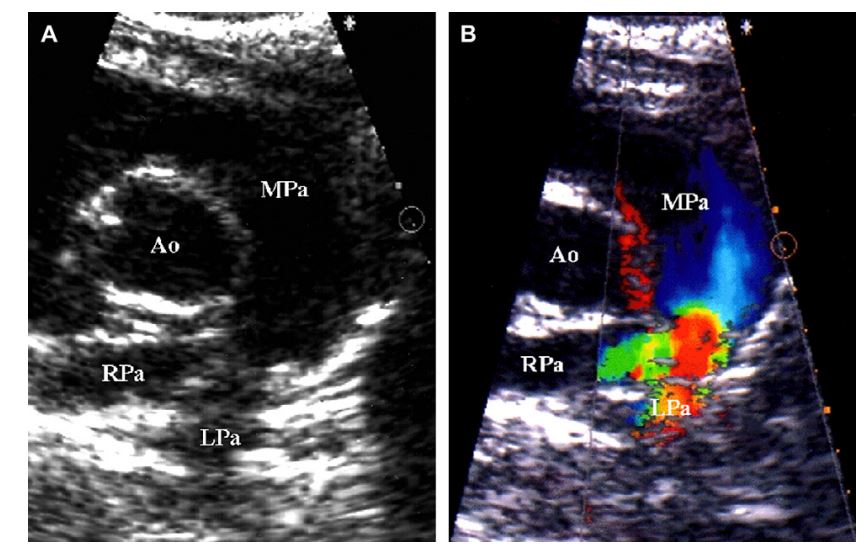
Figure 3 Two-dimensional (A) and color-flow Doppler (B) images of cat 6 with severe pulmonary artery stenosis
Images were obtained during systole. Note the severe stenosis in the body of the pulmonary artery and severe dilation of the main pulmonary artery (A). The stenotic orifice was very difficult to identify using only 2D imaging in this cat. Color-flow Doppler identified the orifice of the stenotic lesion and confirmed flow convergence (B). (Ao = aorta, MPa = main pulmonary artery, RPa = right-branched pulmonary artery, LPa = left-branched pulmonary artery).
All of the cats were alive well into maturity and only cat 6 had died at the time of publication. Cat 6 had been euthanized approximately 15 months after diagnosis due to repeated signs of anorexia, depression, and sneezing, as well as a suspicious pulmonary nodule on radiographs. A necropsy was not allowed. Cat 4 was lost to follow-up at approximately 8 years of age. At the time of publication, serial echocardiograms have been performed on the remaining five cats. No progression in the degree of stenosis has been identified.
Discussion
Pulmonary artery stenosis is a well-known congenital anomaly in humans that most often occurs in conjunction with other congenital cardiac anomalies. The cause of pulmonary artery stenosis is unclear. Postmortem studies in adults reveal intimal invasion by smooth muscle, medial hyperplasia, and increased and disorganized elastin fibers at the site of stenosis. In contrast, postmortem studies in infants reveal fibrous intimal proliferation, medial hypoplasia, and loss of elastin fibers.3 Postmortem samples were not available from cats in this study for comparison with the findings in humans. A classification scheme for pulmonary artery stenosis based on the stenosis location and number of lesions has been developed in humans.1 The classification divides the pulmonary artery into four segments; (1) main pulmonary artery, (2) bifurcation, (3) left- and right-branched pulmonary arteries, and (4) peripheral segmental pulmonary arteries. Single lesions of the main pulmonary artery or branched pulmonary arteries are classified as Type I pulmonary artery stenosis. Type I lesions are further subclassified into Type Ia (isolated stenosis of the main pulmonary artery), Type Ib (isolated stenosis of the right-branched pulmonary artery), and Type Ic (isolated stenosis of the left- branched pulmonary artery). Isolated lesions located at the bifurcation with extension into the proximal branched pulmonary arteries are classified as Type II. Lesions involving the peripheral segmental pulmonary arteries with no abnormalities of the main pulmonary artery, bifurcation, or left- or right-branched pulmonary arteries are classified as Type III. Lesions involving the peripheral segmental pulmonary arteries with additional lesions in the main, left-branched, and/or right- branched pulmonary arteries are classified as Type IV. Type Ia is one of the least common classes of pulmonary artery stenosis seen in humans.1 In contrast, the cats in this study showed no evidence of other concurrent cardiac defects and the lesion was classified as Type Ia in all cats.
Table 2 Echocardiographic findings at initial diagnosis
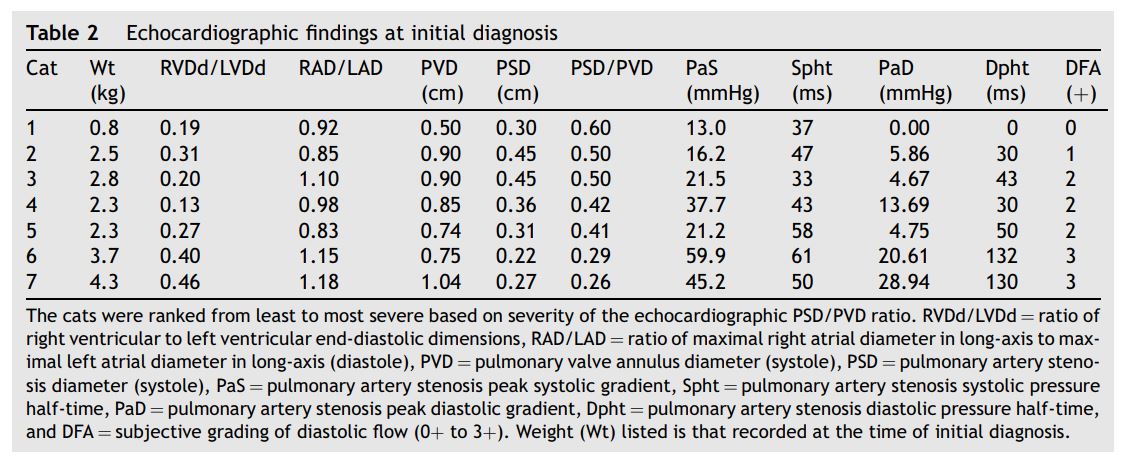
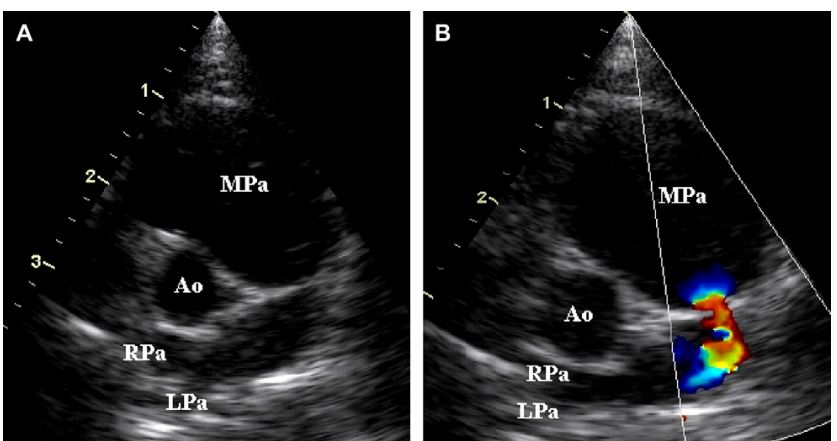
Figure 4 Selective right ventricular angiocardiogram from cat 4 with distal pulmonary artery stenosis
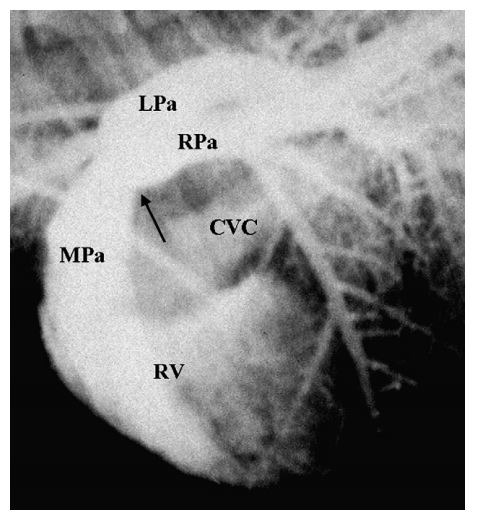
Opacification of the right ventricle (RV), main pulmonary artery (MPa), and right- (RPa) and left (LPa)-branched pulmonary arteries is evident. Mild opacification of the right atrium and the caudal vena cava (CVC) is also present resulting from retrograde movement of the catheter back into the right atrium during injection. Note the stenosis of the main pulmonary artery just proximal to the bifurcation (black arrow) with normal branched pulmonary arteries.
Of the seven cats, six cats were mixed breed, and no sex predilection was suggested (Table 1). The age at the time of diagnosis for most of these cats suggests a congenital lesion although a heritable basis could not be assessed since the majority were stray animals.
All seven cats presented with basilar systolic murmurs although the point of maximal intensity varied (Table 1). Only one cat with severe stenosis and visible Doppler flow throughout diastole had a diastolic murmur. In humans with pulmonary artery stenosis the presence of a diastolic murmur is variable.1,2 Exertional dyspnea was seen in one of the cats with severe stenosis. Exertional dyspnea is one of the most common clinical signs identified in humans with pulmonary artery stenosis, and is likely related to hypoperfusion of the pulmonary arterial tree.3 Based on the blood gas analysis, this was also the likely cause for exertional dyspnea in this cat. It is also possible that certain lesions could result in asymmetric blood flow to branched pulmonary arteries further contributing to ventilation-perfusion mismatch. The available radiographs and ECGs did not reveal specific findings that would differentiate pulmonary artery stenosis from other causes of basilar systolic murmurs. In fact, radiographic or electrocardiographic abnormalities were limited to cats with moderate to severe disease.
Although PaS and PaD were correlated to disease severity, a "natural’’ cut-off between moderate and severe disease was not obvious to the authors (Table 2). Studies in human aortic coarctation support the value of evaluating other diastolic flow parameters.6,8,9 In this study, Dpht and DFA were statistically significant and there appeared to be natural cut-offs between moderate and severe disease using Dpht and DFA. One human paper evaluating patients with aortic coarctation suggested that a Dpht of >100 ms was consistent with severe stenosis.8 Findings in this study suggest that a similar value may be appropriate in cats with pulmonary artery stenosis. Both cats with a Dpht >100 ms and a DFA of 3+ had the most severe stenosis when graded by 2D echocardiography and clinical signs.
The discrepancy between systolic and diastolic Doppler findings and obstruction severity in humans with aortic coarctation is not clear. Causes may be related to changes in cardiac output, collateral blood flow, ductal flow, and the shape and length of the stenosis.6-8 Except for the morphology of the stenosis, none of these would likely be a cause for similar findings in the pulmonary artery. It is possible that, with more severe stenosis, the flexible walls of the main pulmonary artery proximal to the obstruction absorb kinetic energy as the right ventricle contracts against the stenosis. This could result in a lower peak systolic driving force across the stenosis resulting in a lower relative gradient.
The greatest limitations to this study are those inherent to a retrospective study and the small number of cats available. Furthermore, grading of severity was based on 2D echocardiographic findings and clinical signs. Ideally, disease severity would have been graded by angiocardiographic and intra-cardiac pressure data but these invasive diagnostics were believed to not be in the best interest for the majority of these cats.
References
- Gay BB, French RH, Shuford WH, Rogers Jr JV. The roentgenologic features of single and multiple coarctations of the pulmonary artery and branches. Am J Roentgenol Radium Ther Nucl Med 1963;90:599-613.
- D'Cruz IA, Agustsson MH, Bicoff JP, Weinberg M, Arcilla RA. Stenotic lesions of the pulmonary artery. Clinical and hemodynamic findings in 84 cases. Am J Cardiol 1964 April:441 -50.
- Kreutzer J, Landzberg MJ, Preminger TJ, Mandell VS, Treves ST, Reid LM, Lock JE. Isolated peripheral pulmonary artery stenosis in the adult. Circulation 1996;93:1417-23.
- Buchanan JW. Causes and prevalence of cardiovascular disease. In: Kirk RW, Bonagura JD, editors. Kirk's current veterinary therapy XI. Philadelphia: WB Saunders; 1992. p. 647-55.
- Liu SK. Pathology of feline heart disease. Vet Clin North Am 1977;7:323-39.
- Houston AB, Simpson IA, Pollock JC, Jamieson MP, DoigWB, Coleman EN. Doppler ultrasound in the assessment of severity of coarctation of the aorta and interruption of the aortic arch. Br Heart J 1987;57:38-43.
- Marx GR, Allen HD. Accuracy and pitfalls of Doppler evaluation of the pressure gradient in aortic coarctation. J Am Coll Cardiol 1986;7:1379-85.
- Carvalho JS, Redington AN, Shinebourne EA, Rigby ML, Gibson D. Continuous wave Doppler echocardiography and coarctation of the aorta: gradients and flow patterns in the assessment of severity. Br Heart J 1990;64:133-7.
- Valdez-Cruz LM, Cayre RO. Coarctation of the aorta. In: Valdez-Cruz LM, Cayre RO, editors. Echocardiographic diagnosis of congenital heart disease: an embryologic and anatomic approach. Philadelphia: Lippincott-Raven; 1999. p. 475-82.
- Houston AB, Sheldon CD, Simpson IA, DoigWB, Coleman EN. The severity of pulmonary valve or artery obstruction in children estimated by Doppler ultrasound. Eur Heart J 1985;6:786-90.
- Valdes-Cruz LM, Horowitz S, Sahn DJ, Larson D, Oliveria Lima C, Mesel E. Validation of a Doppler echocardiographic method for calculating severity of discrete stenotic obstructions in a canine preparation with a pulmonary arterial band. Circulation 1984;69:1177-81.
- Litster AL, Buchanan JW. Vertebral scale system to measure heart size in radiographs of cats. J Am Vet Med Assoc 2004; 216:210-4.
- Rishniw M, Erb HN. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429-35.
- Boon JA. Acquired heart diseases. In: Boon JA, editor. Manual of veterinary echocardiography. Baltimore: Williams & Wilkins; 1998. p. 261-382.
^Наверх









