Canine idiopathic dilated cardiomyopathy. Part II: pathophysiology and therapy

Author information
Borgarelli M., Tarducci A., Tidholm A., Häggström J. Canine idiopathic dilated cardiomyopathy. Part II: pathophysiology and therapy // Vet J. 2001 Nov;162(3):182-95.
Abstract
Dilated cardiomyopathy (DCM) in dogs is characterized by ventricular and atrial enlargement, and systolic and diastolic dysfunction, with congestive heart failure (CHF) often developing at some stage. With greater understanding of the impact of neuroendocrine stimulation in heart disease, the understanding of the pathophysiology for CHF has changed considerably. It is no longer considered only to be a simple haemodynamic consequence of pump dysfunction, but is now characterized as a complex clinical syndrome with release of many neurohormones, which are believed to have impact on the progression of disease. This change in our understanding of the pathophysiology of CHF has important therapeutic implications. There are strong indications, although not yet proven, that drugs designed to influence the neuroendocrine activity, such as Angiotensin Converting Enzyme (ACE) inhibitors and beta-receptors antagonists, are efficacious as adjunct therapy of heart failure attributable to DCM in dogs. The benefits of drugs designed to influence the myocardial contractile state (positive inotropes) have not been fully evaluated. However, evidence has emerged in recent years indicating that new types of positive inotropes may be beneficial in dogs with DCM. This review focuses on the neuroendocrine aspects of DCM and their possible therapeutic implications and the place for long-term inotropic support in dogs with DCM.
INTRODUCTION
The hallmarks of dilated cardiomyopathy (DCM) are ventricular enlargement, and systolic and diastolic dysfunction, with congestive heart failure (CHF) often (but not invariably) developing at some stage (Wynne & Braunwald, 1997). The rate of progression of DCM is not known. The symptomatic phase is preceded by an asymptomatic phase where several compensatory mechanisms act in concert to maintain cardiac output and prevent CHF (Sisson et al., 2000). These mechanisms operate under the influence of neurohumoral and endocrine factors such as the renin-angiotensin-aldosterone-system (RAAS), endothelin, catecholamines and vasopressin to compensate the homeostatic imbalances created by the failing heart (Nicholls et al., 1996). However, the actions of these systems, which are intended to provide a beneficial adaptation in CHF, are frequently inappropriately activated, leading to fluid retention and increased peripheral vascular resistance, which may have negative long-term effects on the failing heart in the form of an increased cardiac workload (Nicholls et al., 1996).
The RAAS acts in order to maintain normal arterial pressure by expanding plasma volume (sodium and water retention), arteriolar vasoconstriction (increasing afterload) and eliciting thirst (Packer, 1987a; Lumbers, 1999). The augmented levels of plasma catecholamines, in particular the release of norepinephrine by adrenergic cardiac nerves, increase both myocardial contractility (positive inotropic effect) and heart rate (positive chronotropic effect). This response, on the other hand, also leads to an increased cardiac workload and may accelerate the rate of myocardial cell death (Unger, 2000). Fortunately, actions of these vasoconstrictive-fluid retentive systems are counteracted by vasodilator, diuretic and natriuretic factors such as the natriuretic peptides and adenosine (Wei et al., 1993; Funaya et al., 1997). Indeed, it is well accepted that congestive heart failure in dogs with dilated cardiomyopathy may be described as a condition of generalized neurohormonal activation and parasympathetic withdrawal (Floras, 1993)
Presumably, the neuroendocrine profile varies depending on different cardiac disorders, phase and severity of heart failure (Unger, 2000). In particular, the actions of the sympathetic system and RAAS appear to be strictly interrelated at many levels (Cody, 1997). Moreover many, if not all, of the mediators involved in the control of the cardiovascular system in heart failure have effects on the growth of cardiovascular tissue and may thereby play an important role in the ‘remodelling’ of the myocardium and vasculature (Colucci & Braunwald, 1997).
In the few last years, many studies have focused on the neuroendocrine profile of various cardiac diseases and it is now clear that activation of neurohumoral systems contribute to the progression of signs of heart failure (Kluger et al., 1982). This increased neuroendocrine activity might have therapeutic implications. Definitions, clinical characteristics, aetiology, epidemiology, pathogenesis and pathological features of dilated cardiomyopathy have been discussed in the first part of this review (Tidholm et al., 2001).
The objective of this second part is to focus on the neuroendocrine aspects of the disease and their possible therapeutic implications and the place for long-term inotropic support in dogs with dilated cardiomyopathy.
PATHOPHYSIOLOGY
Increased sympathetic activity. During the last two decades, it has become apparent that CHF is accompanied by an elevation in circulating cathecolamines, particularly norepinephrine (NE) (Packer et al., 1987b). Indeed, the severity of left ventricular dysfunction and mortality are directly correlated with the extent of increased plasma NE concentration. However, it is not clear if there is a causal relationship between the increased circulating NE and the increased mortality, or if the increased concentrations simply reflect the severity of congestive heart failure (Thomas & Marks, 1978; Cohn et al., 1984).
In symptomatic dilated cardiomyopathy, plasma catecholamine concentrations have been found significantly higher in both human beings (Yamada et al., 1996; Floras et al., 1991; Bristow, 1993; Floras, 1993) and dogs (Ware et al., 1990; Re et al., 1999; Borgarelli et al., 1999a).
Increased concentrations of levels of plasma catecolamines have also been found in human patients with asymptomatic left ventricular dysfunction (Viquerat et al., 1985; Francis et al., 1990; Floras et al., 1991) and in asymptomatic DCM dogs (Borgarelli et al., 1999a). The increased concentrations of plasma NE may be attributed to increased release from adrenergic nerve endings and the consequent ‘spillover’ into the plasma (Rose et al., 1985; Hasking et al., 1986). It may also be attributed to reduced uptake by adrenergic nerve endings (Liang et al. 1989). In human beings the adrenergic activity appears to be correlated not only to symptomatology but also to physiological indices of severity of heart failure, such as left and right atrial filling pressures (Leimbach et al., 1986).
The increased adrenergic activity has several adverse effects on cardiac structure and function, which include downregulation of myocardial 1- adrenoreceptor (AR) density, decreased -adrenergic responsiveness to endogenous or exogenous agonists, trophic and toxic effects on cardiac myocytes, exacerbation of arrhythmias and impairment of ventricular diastolic and systolic functions (Simpson et al., 1983; Floras, 1993; Mann et al., 1992; Clark et al., 1993). It has been demonstrated that within 24–72 h of increasing adrenergic drive, the 1 – AR of the heart undergo downregulation, and cellular response to catecholamines wanes rapidly (desensitization) (Bristow, 1984; Vatner et al., 1985; Hausdorff et al., 1990; Kittleson, 1994). -AR desensitization occurs as a result of two different alterations in their signalling, involving first reduced numbers of receptor (receptor downregulation) followed by an impairment in the function of the remaining receptors (receptors uncoupling) (Dzimiri, 1999). Interestingly, the downregulation of -AR in asymptomatic and symptomatic dilated cardiomyopathy dogs was not associated with alteration in receptor affinity characteristics (Re et al., 1999).
TABLE I Lymphocyte distribution of adrenoreceptor subtypes in normal and dilated cardiomyopathy dogs
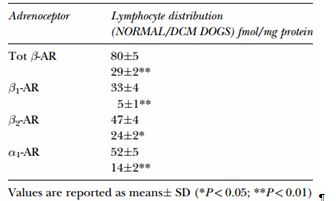
In addition to the symptomatology, the cause of heart failure may also influence the degree of sympathetic activation. One study reported that mean NE concentration in dogs with dilated cardiomyopathy was greater than in dogs with mitral valve disease, which may indicate more severe decompensation and neurohumoral activation (Ware et al., 1990).
Adrenoreceptor distribution and function in normal and dilated cardiomyopathy dogs have recently been investigated. The presence of 1-AR and -AR subtypes was demonstrated, as in other mammalian species, both in canine myocardial and lymphocyte cell membranes (Re et al., 1999). In the normal dog, circulating lymphocytes have a population of 1 and 2 AR almost equally represented (Table I). By contrast, 2-AR predominate in human lymphocytes (Brodde, 1991; Yamada et al., 1996). A significant correlation between cardiac and lymphocyte AR concentration has been described (Re et al., 1997; Tarducci et al., 1997). Beta-1 AR downregulation in dogs with DCM has been demonstrated in myocardial cells and in circulating lymphocytes, not only in the symptomatic but also in the asymptomatic phase. In one study, dogs with symptomatic dilated cardiomyopathy and occult DCM had significantly lower density of total AR, 1- and 2-AR subtypes on their lymphocytes as compared to the control group (Borgarelli et al., 1999a).
In humans, downregulation of -AR has been associated with an increased activity of -AR (Dzimiri, 1999) which leads to a weak inotropic response mediated through the increased activity of phospholipase C (Landzberg et al., 1991; Bristow, 1993). It is possible that the malfunction of -AR acts as a ‘switch’ to turn on -AR but, at present, the exact mechanism for -adrenergic upregulation is still to be elucidated (Dzimiri, 1999). Myocyte hypertrophy, re-expression of foetal genes and the induction of peptide growth factor were also correlated with stimulation of the 1-AR receptor in rats (Bisphoric et al., 1987; Takahashi et al., 1994). In contrast to 1, the number of myocardial 1-AR remains unchanged in human heart failure (Bristow et al., 1988). A different response was observed both in lymphocyte and myocardial cell membranes of dilated cardiomyopathy -affected dogs, where all subtypes (total, 1-, 2- and 1-AR) appear to be downregulated (Re et al., 1999).
Renin-angiotensin-aldosterone system It is now widely accepted that congestive heart failure is associated with an increased RAAS activity in dogs and humans (Kluger et al., 1982; Cody & Laragh, 1988; Colucci & Braunwald, 1997). It is well known that systemic activation of the RAAS increases retention of sodium and water, leads to arteriolar vasoconstriction and elicits thirst (Lumbers, 1999). However, the major portion (90 to 99%) of angiotensin-convertingenzyme (ACE) in the body is found in the tissues (Dzau & Re, 1994; Hirsch et al., 1991; Huang et al., 1994) and a myocardial renin-angiotensin system (RAS) with local generation of Angiotensin II (AII) has been reported by several authors (Unger et al., 1987; Foult et al., 1989). This local RAS has been suggested to be the most significant pathway of myocardial AII production (Malik et al., 1997), although other possible pathways have been hypothesized, i.e. chymase (Dell’Italia et al., 1995).
Indeed, the concentration of AII is considerably higher (approximately 1000 times) in myocardial tissue than in plasma (Dell’Italia et al., 1995).
Recent studies have shown that an increased activity of local (e.g. myocardial) renin-angiotensin-aldosterone-system occurs early in the course of experimentally induced heart failure and that these systems may play a major role in the progression of heart failure (Hirsch et al., 1991; Foult et al., 1989). At the local level, increased RAAS activity may lead to modification of the myocytes and myocardial architecture (mainly through the action of AII and aldosterone) by inducing myocyte hypertrophy, myocyte necrosis (Tan et al., 1991) and fibrosis, the latter mediated through increased collagen synthesis (cardiac remodelling). These changes lead eventually to systolic and diastolic dysfunction (Brilla & Rupp, 1994; Wilke et al., 1996). Myocardial stretch is believed to activate the synthesis of AII and other RAS components (Danilo et al., 1999; Malhotra et al., 1999). Furthermore, an upregulation of the AII receptor gene associated with myocyte hypertrophy and fibrosis has been reported in humans with dilated cardiomyopathy (Ohtani et al., 1997).
Suppression of myocardial ACE-activity leads to decreased levels of AII, but the trophic effect of AII may still prevail through an upregulation of AIIreceptors (Dell’Italia et al., 1995). However, it should be remembered that the majority of these studies were conducted in laboratory animal models of heart failure, which may not be equivalent to naturally occurring disease.
Recently, two studies have investigated the activity of renin-angiotensin-aldosterone-system in plasma in both symptomatic and asymptomatic dogs with DCM (Koch et al., 1995; Tidholm et al., 2000). One study found that both plasma renin activity (PRA) and plasma aldosterone concentration (PAC) were increased in symptomatic dilated cardiomyopathy dogs (Koch et al., 1995). PRA was also increased in some dilated cardiomyopathy asymptomatic (NYHA class I) dogs too, while PAC was not. These data have been confirmed in another study that found PRA and PAC significantly increased in symptomatic DCM dogs, compared to asymptomatic dilated cardiomyopathy and to normal dogs (Tidholm et al., 2000). However, the lack of activation of systemic renin-angiotensin-aldosterone-system does not preclude the possible activation of the local systems (i.e. myocardial RAS).
NATRIURETIC PEPTIDES
Investigations have demonstrated the existence of a family of structurally related peptides, the atrial (ANP), brain (BNP) and C-type (CNP) natriuretic peptides (Yasue et al., 1989; Arbustini et al., 1990; Thibault et al., 1999). These peptides participate in the integrated control of renal and cardiovascular function (Wei et al., 1993). ANP is a 28-amino acid peptide that is secreted from both cardiac atria in response to atrial stretch (Goetz, 1988; Hare et al., 1991), increased atrial pressure (Hare et al., 1991), endothelin, adrenergic stimulation (Thibault et al., 1999) and tachycardia (Cogan, 1990a). It possesses natriuretic, vasoactive and vasopressin- renin- and aldosterone-inhibiting actions (Cogan, 1990b; Clark et al., 1991). BNP is a 32-amino acid peptide, also found in the heart with structural and biological activities similar to those of ANP (Itoh et al., 1988; Grantham et al., 1997). It is stored mainly in the cardiac ventricular myocardium and may be responsive to changes in ventricular filling pressure (Grantham et al., 1997). CNP is a 22-amino acid peptide primarily localised in endothelial cells throughout the vascular system (Wei et al., 1991). It is believed to act in a paracrine manner and, therefore, to be primarily involved in local control of the vasculature. However, its role has still to be clarified. ANP, BNP and CNP decrease cardiac preload and suppress renin, aldosterone and vasopressin secretion (Kawai et al., 1996), but unlike ANP and BNP, CNP is not natriuretic (Wei et al., 1993; Struthers, 1994; Colucci & Braunwald, 1997).
In CHF, the plasma concentrations of ANP are increased in response to increased atrial stretch and increased heart rate (Goetz, 1988; Hare et al., 1991; Cogan, 1990a), whereas circulating BNP increases primarily in response to ventricular dysfunction and dilatation (Wei et al., 1993). CNP elevation, unlike ANP and BNP, is not characteristic of CHF (Chen & Burnett, 1998). Earlier studies have shown different secretion patterns between ANP and BNP peptides depending on the type of cardiac disease. Indeed, BNP concentrations were considerably higher in human patients with DCM than in those suffering from mitral valve stenosis (Yoshimura et al., 1993) and ANP levels increased between three and four times in human patients with dilated cardiomyopathy compared to patients with left-sided valvular heart disease (Haass et al., 1988; Saito et al., 1987). These differences may be due to the distribution of the two peptides in the atria and ventricles and different stimuli for secretion of the two peptides into the circulation.
However, a disturbed peripheral metabolism of ANP and resistance to its biological effects has also been demonstrated in human patients with dilated cardiomyopathy (Clerico & Iervasi, 1995).
In dogs with dilated cardiomyopathy, ANP has also been reported to be increased three to four times as compared to normal individuals (Vollmar et al., 1991). One study focussing on DCM found a significant elevation of the N-terminal portion of ProANP (1-98) (NTproANP) (which is secreted in equimolar concentrations with ANP but has a longer half-life in plasma and better in vitro stability) in symptomatic dilated cardiomyopathy dogs as compared to dogs with asymptomatic DCM and to normal control dogs (Tidholm et al., 2000). The NT-proANP concentrations were not significantly higher in asymptomatic dogs compared to control dogs, but the variations were greater in dilated cardiomyopathy dogs and values were found to be particularly high in dogs with atrial fibrillation. These results are in accordance with studies of human DCM where a positive correlation exists between ANP and the degree of ventricular impairment and dilatation (Arbustini et al., 1990). Less is known about BNP in canine dilated cardiomyopathy. The reason for this may be that measurement of BNP is often more difficult because its amino-acid sequence is considered to be poorly conserved across species (Lang et al., 1992).
More recent studies reveal strong homology between mature forms of BNP in dogs, cows, pigs and sheep (Aitken et al., 1999). Nevertheless, recently presented preliminary data on plasma BNP in some cardiac diseases in the dog suggests increased concentrations of BNP in early stages of heart disease (Sisson, 1999). Measurement of BNP in plasma might, in the future, be a useful tool for therapeutical decisions (Ha¨ggstrom et al., 2000). The natriuretic peptides may not only be important as markers for cardiac disease but may also be of functional importance in limiting the activity of the renin-angiotensin-aldosterone-system in early decompensated congestive heart failure. Thus, future clinical trials involving drugs that interact with the natriuretic peptides, such as the neutral endopeptidase inhibitors (Chen & Burnett, 1999), may show beneficial effects in dogs with dilated cardiomyopathy.
THYROID HORMONES
The thyroid hormones play an important part in cardiac performance as increasing concentrations of triiodothyronine (T3) exert positive inotropic and chronotropic effects (Dillman, 1990). The thyroid hormones increase cardiac concentration of cyclic AMP and inositol-1,4,5-tris phosphate (InsP3). In this way, more calcium enters the cytosol, augmenting the contractility, but cellular overload of calcium during diastole may impair relaxation. Moreover, myocardial cells overloaded with calcium are more susceptible to arrhythmias. In human patients with severe DCM and down regulation of -AR, the elevation of serum ionised calcium has been shown to augment contractility (Ginsburg et al., 1983).
Triiodothyronine can also alter the relationship between the sympathetic nervous system and the cardiovascular system by increasing sympathetic activity or enhancing responsiveness of the myocardium to adrenergic stimuli by increasing the number of -AR (Rutherford et al., 1979) Some studies have indicated a higher prevalence of hypothyroidism in dogs with dilated cardiomyopathy, particularly in the Doberman Pincher breed (Panciera, 1994). Accordingly, it has been suggested that hypothyroidism may play a role in the development of DCM in dogs, although most studies fail to identify any such relationship (Calvert et al., 1982, 1998). In another study, thyroid-stimulating hormone (TSH) and total thyroxine (TT4) did not differ significantly between normal, asymptomatic and symptomatic DCM dogs, whereas significantly lower concentrations of free thyroxine (FT4) were found in symptomatic DCM dogs compared with asymptomatic dilated cardiomyopathy dogs and normal dogs (Tidholm et al., 2000). Concentrations of FT4 may be regarded as being more accurate than TT4 in detecting hypothyroid states, but, in general, a TSH stimulation test is needed to verify the diagnosis if results of baseline TT4, FT4 or TSH are discordant (Chastian & Panciera, 1995).
Due to the fact that serum concentrations often decrease in dogs as a result of concurrent illness, i.e. ‘sickeuthyroidsyndrome’(Panciera,1999;Dixonetal., 1999), such as CHF, and that a concentration of biologically active hormone T3 does not accurately reflect thyroid hormonal status in the dog (Feldman & Nelson, 1987), the value of determining serum concentrations of thyroid hormones is limited in this species. Recently, mRNA coding for the four isoforms of the thyroid hormone receptor were investigated in dogs with symptomatic DCM or chronic valvular heart disease (CVD). The results showed that the subtypes 1 and 2 were equally upregulated in both diseases as compared to normal individuals, suggesting that the upregulation was attributable to the development of congestive heart failure and not the primary disease (Shahrara et al., 1999). Studies concerning thyroxine therapy in human patients with DCM report of an improved cardiac function (Moruzzi et al., 1996), and experimental studies in dogs with pacinginduced cardiomyopathy report of positive inotropic and lusitropic effects ( Jamall et al., 1997). However, the therapeutic value of thyroxine in dilated cardiomyopathy has not yet been evaluated in dogs with naturally occurring disease.
THERAPY
The therapy for DCM dogs with overt heart failure may include an ACE-inhibitor, one or more diuretic agent and cardiac glycosides (Sisson & Thomas, 1995). However, studies have recently reported beneficial effects of inotropic agents, ACE-inhibitors and -receptor antagonists. In this paper, we will review the use of inotropic support, ACE inhibitor and -receptor antagonist therapy in DCM.
INOTROPIC DRUGS
As DCM is characterized by a primary systolic dysfunction, there is some rationale in using inotropic support to treat the disease. Pimobendan, amrinone and milrinone are three non-digitalis inotropic drugs that have been used to treat dogs with systolic dysfunction both in experimental and clinical trials (Braunwald, 1985; Kittleson et al., 1985; Treadway, 1985; Luis Fuentes et al., 1998; Lombard, 2000; Kubo et al., 1992; Lubsen et al., 1996). These drugs exert positive inotropic as well as peripheral vasodilating effects (Fujino et al., 1988). The inotropic activity of pimobendan, amrinone and milrinone is caused by an inhibition of the cAMP-phosphodiesterase type III (Alousi et al., 1983). Thus, their inotropic effect appears as a consequence of increased intracellular levels of cAMP (Fujino et al., 1988).
The inotropic effect remains unchanged over time with respect to sympathomimetic agents because the actions of amrinone and milrinone are not dependent on beta-receptors (Kittleson & Kienle, 1998).
The increase in contractility observed with amrinone and milrinone seems to be species-dependent, with maximum effect detected in dogs and cats compared to humans and rats (Kittleson & Kienle, 1998). Both compounds have similar pharmacological effects and are marketed for intravenous administration only, while clinical studies have been performed on the effects of chronic oral administration of milrinone. In a large study in dogs with heart failure, involving 117 dogs with DCM, this drug did not influence survival (Keister, 1988); moreover, the short duration of action necessitates oral administration several times a day. On the contrary, in human patients with heart failure, chronic administration of oral milrinone resulted in an increased risk of sudden death (Packer et al., 1991; Remme, 1993). It appears that an increased workload on an already diseased myocardium, such as in the case of DCM, may decrease longevity.
Pimobendan is a new drug that possesses both inotropic and vasodilating properties and recently has been evaluated both in human and veterinary studies (Luis Fuentes et al., 1998; Lombard, 2000; Kleemann et al., 1998). The drug acts by inhibiting cAMP-phosphodiesteraseIII(similartomilrinoneand amrinone), and by increasing the calcium sensitivity of cardiac myofibrils with a proportional increase in ATPase activity (Fritsche et al., 1986). This means that myocardial contractility is enhanced without any increase of myocardial oxygen consumption as occurs with sympathomimetics and pure phosphodiesterase inhibitors (Fujino et al., 1988). Moreover, a marked reduction of pulmonary capillary wedge pressure, an increase in cardiac output, stroke volume, left ventricular systolic pressure gradient (dp/dt) and renal blood flow in a dose-dependent manner were demonstrated in various experimental models of impaired myocardial function in dogs, while heart rate, systolic and diastolic blood pressures and myocardial oxygen consumption were virtually unaffected (Pouleur et al., 1988; Van Meel, 1989).
In human patients, two double-blind, placebocontrolled, multicentre studies evaluated pimobendan in 189 and 317 (PICO trial) heart failure patients respectively (Kubo et al., 1992; Lubsen et al., 1996). Both these trials reported a significant improvement of exercise tolerance and no proarrhythmic effects; however, the PICO trial reported that in the pimobendan group the mortality rate tended to be (not significantly) higher than in the placebo group (Lubsen et al., 1996). No significant differences were found between placebo and pimobendan groups regarding plasma norepinephrine concentrations (Kubo et al., 1992). One preliminary study conducted on 81 dogs with dilated cardiomyopathy has shown that treatment with pimobendan or combined therapy with pimobendan and benazepril appears to exert a significant positive influence on symptoms of heart failure compared to treatment with benazepril alone (Lombard et al., 2000). In another study, increased survival times were detected in a group of Dobermans affected with DCM treated with conventional therapy and pimobendan compared to the placebo group (which received conventional therapy alone). However, the same study failed to show any effect on survival in a group of Cocker Spaniels with DCM treated with the same protocol (Luis Fuentes et al., 1998).
DIGITALIS GLYCOSIDES
Cardiac glycosides are thought to posses a weak inotropic effect and negative chronotropic and antiarrhythmic effects. Therefore, the indications for their use in human patients are systolic dysfunction and elevated heart rate, in particular supraventricular tachyarrhythmias. (Gavaghan, 2000). By interfering with Na‡,K‡-ATPase in the myocardium, digitalis indirectly increases the number of calcium ions within the cells. This mechanism works well in normal cells, while in failing myocardium this effect appears to be reduced (Mason et al., 1972). Moreover, the risk of a pro-arrhythmic effect in calciumoverloaded cells must be considered in patients with ventricular tachyarrhythmias. Other reported effects of digitalis glycosides include diuresis and restoration of baroreceptor function. The diuretic effect appears to be related to natriuresis, as recently described in rats (Wald et al., 1991).
The reset of the baroreceptor function leads to a reduction in plasma catecholamine concentrations, sympathetic nerve activity and PRA in human studies (Ferguson, 1992) Cardiac glycosides have not been shown to reduce overall mortality rates in a large placebo-controlled trial involving human heart failure patients, although mortality due to heart failure was reduced (The Digitalis Investigation Group, 1997). The PROVED (Uretsky et al., 1993) and RADIANCE (Packer et al., 1993) trials indicated that the efficacy of digoxin may be dose dependent, as better results seem to be associated with lower plasma concentrations than previously recommended. These trials did not show further clinical improvement at serum concentrations higher than 0.5 to 0.9 ng/ml (Uretsky et al., 1993; Packer et al., 1993; Slatton et al., 1997). There are few studies evaluating digitalis efficacy on survival in dogs with DCM. In one trial, dogs with DCM that responded to digoxin by increased contractility, i.e. fractional shortening, lived significantly longer than did those that did not respond (Kittleson, 1986). In this report, the author speculated that the lack of response to digoxin in some dogs may have been caused by an altered intracellular handling of calcium that may be present in the myocytes as a consequence of damaged intracellular structures, e.g. sarcoplasmatic reticulum.
ACE-INHIBITORS
The use of ACE-inhibitors has become widespread in veterinary medicine since the two large human studies CONSENSUS (CONSENSUS Trial Group, 1987) and SOLVD (SOLVD Investigators, 1991) showed an increased survival in patients with congestive heart failure by adding the ACE-inhibitor enalapril to conventional therapy. Currently four placebo-controlled, doubleblind studies have been published regarding the efficacy of therapy and its effects on survival in dogs with CHF caused by DCM or CVD (COVE Study Group, 1995; IMPROVE Study Group, 1995; Ettinger et al., 1998; BENCH study, 1999). The IMPROVE study and the COVE study showed an overall clinical improvement in patients treated with enalapril as compared to placebo-treated dogs. This is in agreement with the data from human studies (COVE Study Group, 1995; IMPROVE Study Group, 1995).
Interestingly, if data from dogs with dilated cardiomyopathy and CVD disease are considered separately, dogs with DCM appeared to benefit more than dogs with CVD (COVE Study Group, 1995; IMPROVE Study Group, 1995), suggesting possible differences in degree of the renin-angiotensin-aldosterone-system activity between the two diseases.
In contrast, the LIVE study showed that enalapril therapy in combination with standard treatment (diuretics with or without digoxin) increased the number of days to treatment failure in CVD dogs whereas only a tendency was found in DCM dogs (P ˆ 0.06) (Ettinger et al., 1998). Indeed, the number of days to treatment failure was similar in the CVD and dilated cardiomyopathy dogs in both the enalapril and placebo groups.
Another study investigating the efficacy and tolerability of long-term administration of the ACEinhibitor benazepril has been recently published (BENCH study, 1999). In this study, no beneficial effects were observed on survival in the DCM treatment group, whereas an increased survival was statistically evident in all dogs included the study and in the subgroup of dogs with CVD.
Today, there is a general recommendation for the use of an ACE-inhibitor in all human patients with left ventricular systolic dysfunction, irrespective of symptoms (Committee on Evaluation and Management of Heart Failure, 1995). This recommendation is based on several large trials that showed beneficial effects in terms of improved quality of life, decreased hospitalisation time and increased overall survival in people with asymptomatic heart disease or people with no evidence of cardiac disease but belonging to a risk group for developing heart disease (The SOLVD Investigators, 1992; The HOPE Investigators, 2000). The beneficial effects of ACE-inhibitors in asymptomatic and/or subclinical (occult) DCM in dogs are less clear. There are no prospective veterinary studies available that evaluate the efficacy of enalapril in these dogs. In a retrospective study, O’Grady et al. (1997) reported a significant delay in the onset of clinical signs in dogs treated with an ACE-inhibitor compared to dogs which did not receive the drug. Furthermore, the only indication of increased circulating RAAS activity (indirectly suggesting beneficial effects of ACE inhibitors) in DCM dogs is, at present, a study in which the asymptomatic DCM dogs had an increased urinary aldosterone to creatinine ratio as compared to normal control dogs (Tidholm et al., 2000). However, no significant differences were found in PRA and PAC in asymptomatic dilated cardiomyopathy (Koch et al., 1995; Tidholm et al., 2000). In this context, it should be remembered that differences regarding the degree of activation of RAAS have been found between breeds, between different diseases and between individuals (Pedersen et al., 1995a,b; Koch et al., 1995).
An interesting argument for early intervention with an ACE-inhibitor in DCM dogs is the observation that it may counteract -AR downregulation or, alternatively, increase -AR upregulation by inhibiting the angiotensin II activity (Dzimiri, 1999). In clinical settings, it has also been observed that the administration of ACE-inhibitors can increase cardiac and peripheral 2-AR levels as well as improving prognosis and cardiac function in human patients with CHF (Gilbert et al., 1993). Nevertheless, therapeutic decisions on whether to treat dogs with asymptomatic DCM with ACE inhibition should rest on controlled clinical trials rather than the analysis of systemic RAAS activity.
BETA-ADRENOCEPTOR ANTAGONISTS
Currently the major indication for using this class of drug is to block the long term toxic and growthpromoting effects of norepinephrine (Bristow et al., 1996). The exact mechanisms through which the beneficial effects of the -adrenergic antagonist are mediated in heart disease are not known. As mentioned before, the -AR is downregulated, and -adrenoceptor antagonists have been shown to reverse this trend in humans (Andersson et al., 1996) and dogs (Borgarelli et al., 1999b). As -AR density during beta-blockade tends to return toward normal levels, it has been hypothesized that the adrenergic system could then participate in inotropic support during periodic increases in local neurotransmitter release or elevation of circulating catecholamines produced by exercise or other activities, despite the presence of a competitive betablocking agent (Andersson et al., 1996). Evidence also suggests that chronic beta-blockade may enhance coupling of the beta-receptor to the guanine nucleotide stimulatory protein (Feldman, 1993). This could be an additional benefit of chronic beta-blockade in patients with CHF.
Several studies on humans have shown beneficial effects of -blockade on symptomatology, systolic function and survival in patients with dilated cardiomyopathy (Waagstein et al., 1993; CIBIS Investigators Committee, 1994; Packer et al., 1996; Bristow et al., 1996).
Clinical effectiveness has been documented for many -receptor antagonists including carvedilol, metoprolol, bucindolol and bisoprolol in combination with standard therapies (Fowler, 1998). Furthermore, the finding that the sympathetic nervous system appears to be activated early in the course of the disease suggests that -adrenergic antagonist therapy should be initiated early in the course of the disease. When deciding which kind of -receptor antagonist (i.e. selective vs. non-selective) should be preferred, there are some considerations to take into account. It is important to recognize that as heart failure progresses, the proportion of 2- and 1- receptors in the heart increase and the oxidative stress is greatly enhanced. Heart failure in DCM is consequently associated with alterations in all AR subtypes (Packer, 1997). Moreover, there appears to be qualitative and quantitative differences in patient responses to selective vs. non-selective -receptor antagonists. For example, the administration of a selective -receptor antagonist has been associated with an increase in NE spillover mainly from 2-ARs, whereas the use of a non-selective drug appears to lead to a reduction in this spillover (Newton & Packer, 1996; Dzimiri, 1999). These considerations suggest that non-selective -AR blockade should be preferred in order to alleviate the cardiac effects of increased adrenergic activity.
Carvedilol is a ‘third generation’ non-selective -receptor antagonist agent that has some mild alphaadrenergic antagonistic effects. This drug has been tested in several recent human studies and it has been shown to exert a significant amelioration in left ventricular function, clinical symptoms and survival (Packer et al., 1996). However these studies included only few patients with severe congestive heart failure (NYHA IV), and there are conflicting data regarding the use of -receptor antagonist therapy in severe heart failure patients (Packer, 1997; Eichorn & Stevenson, 1998; Macdonald et al., 1999). Few reports describe the use of -receptor antagonist therapy for treatment of dogs with congestive heart failure. In one study, dogs with experimentally induced heart failure using intra-coronary injections of microspheres showed a stabilization of the left ventricular function following the administration of metoprolol, a selective 1-receptor antagonist, compared to the control group (Sabbah et al., 1994).
In another study, atenolol, another selective 1-receptor antagonist, was administered to a group of dogs with experimentally induced mitral regurgitation, resulting in improvement of myocardial function (Tsutsui et al., 1994). Again, these studies involved models of heart failure which may not reflect the natural course of the disease. Some reports involving few cases describe the unsuccessful results of -receptor antagonist treatments in dogs with dilated cardiomyopathy and overt heart failure (Kittleson & Kienle, 1998).
The authors speculate that the lack of treatment success in dogs (as opposed to in humans) could be attributable to more a rapid progression of the disease and more severe heart failure in this species. It is important to remember that the initial pharmacological response to selective -receptor antagonist administration is represented by a decrease of adrenergic support to the heart with possible exacerbation of symptoms. Therapy should begin in stable patients with low doses, and the drug up-titrated very slowly (Eichorn & Stevenson, 1998; Jessup & Brozena, 2000). The beneficial effects of -adrenergic antagonist therapy in DCM do not necessarily become apparent until after long-term medication (Waagstein et al., 1989). As there is mounting evidence that -receptor antagonist therapy exerts many beneficial effects in human beings with class II and III NYHA CHF, controlled clinical trials should also be encouraged in veterinary medicine to evaluate the efficacy and the safety of -receptor antagonist therapy in dogs with cardiac diseases.
CONCLUSIONS
DCM represents a complex clinical syndrome involving many pathophysiological mechanisms. With greater understanding of the impact of neuroendocrine stimulation in the developing symptoms of heart failure, the importance of a therapy designed to influence the neuroendocrine activity has become clear. So far, some studies have shown the positive effects on signs of heart failure of the therapy with ACE-inhibitor in patients with symptomatic disease.
At present, therapy of symptomatic patients remains mainly a therapy designed to control the symptoms of heart failure (i.e. diuretics, digoxin and vasodilators). Regardless of type of therapy instituted, the prognosis of symptomatic DCM is grave. It is not unlikely that certain types of therapy, i.e. drugs interacting with the neuroendocrine systems and certain positive inotropes, may have influence of the progression of DCM should therapy have been instituted earlier. Therefore, clinical studies are needed in asymptomatic patients, not only symptomatic. To our knowledge, there is presently only one study exploring effects of ACE-inhibitors in asymptomatic canine patients.
REFERENCES
- AITKEN, G. D., RAIZIS, A. M., YANDLE, T. G., GEORGE, P. M., ESPINER, E. A. & CAMERON, V. A. (1999). The characterization of ovine genes for atrial, brain, and C-type natriuretic peptides. Domestic Animal Endocrinology 16, 115–121.
- ALOUSI, A. A., CANTER, J. M., MONTENARO, M. J., FORT, D. J. & FERRARI R. A. (1983). Cardiotonic activity of milrinone, a new and potent cardiac bipyridine, on the normal and failing heart of experimental animal. Journal Cardiovascular Pharmacology 5, 792–903.
- ANDERSSON, B., CAIDHAL, K., DI LENARDA, A., WARREN, S. E., GOSS, F., WALDENSTROM, A., PERSSON, S., WALLENTIN, I., HJALMARSON, A. & WAAGSTEIN, F. (1996). Changes in early and late diastolic filling patterns induced by longterm adrenergic -blockade in patients with idiopathic dilatated cardiomyopathy. Circulation 94, 673–682.
- ARBUSTINI, E., PUCCI, A., GRASSO, M., DIEGOLI, M., POZZI, R., GAVAZZI, A., GRAZIANO, G., CAMPANA, C., GOGGI, C. & MARTINELLI, L. (1990). Expression of natriuretic peptide in ventricular myocardium of failing human hearts and its correlation with the severity of clinical and hemodynamic impairment. American Journal of Cardiology 66, 973–998.
- THE BENCH (BENazepril in Canine Heart disease) STUDY GROUP (1999). The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multicentric, prospective, randomized, double blinded, placebo-controlled, longterm trial. Journal of Veterinary Cardiology 1, 7–18.
- BISPHORIC, N. H., SIMPSON, P. C. & ORDHAL, C. P. (1987). Induction of the skeletal alpha-actin gene in alpha1- adrenoceptor-mediated hypertrophy of rat cardiac myocytes. Journal of Clinical Investigation 80, 1194–1199.
- BORGARELLI, M., BADINO, P., BERGAMASCO, L., BUSSADORI, C., ODORE, R., RE, G., TARDUCCI, A. & DOTTA U. (1999a). Lymphocyte -adrenoceptor downregulation in Great Danes with occult dilated cardiomyopathy (DCM) and with DCM and heart failure. The Veterinary Journal 158, 128–134.
- BORGARELLI, M., BADINO, P., BERGAMASCO, L., ODORE, R., RE, G., TARDUCCI, A., ZANATTA, R. & BUSSADORI, C. (1999b). Lymphocyte adrenoceptor downregulation DCM dogs. Proceedings of 17th American College of Veterinary Internal Medicine Forum 10–13 June, Chicago, 90–92.
- BRAUNWALD, E. (1985). A symposium: amrinone-introduction. American Journal Cardiology 56, 1B–2B. BRILLA, C. G. & RUPP, H. (1994). Myocardial collagen matrix remodeling and congestive heart failure. Cardiologia 39, 389–393.
- BRISTOW, M. R. (1984). Myocardial -adrenergic receptor down regulation in heart failure. Internal Journal of Cardiology 5, 648–652.
- BRISTOW, M. R., MINOBE, W., RASMUSSEN, R., HERSHBERGER, R. E. & HOFFMAN, B. B. (1988). Alpha1-adrenergic receptors in the nonfailing and failing human heart. Journal of Pharmacology and Experimental Therapeutics 247, 1039–1045.
- BRISTOW, R. M. (1993). Changes in myocardial and vascular receptors in heart failure. Journal of American College Cardiology 22 (Suppl. A), 61A–71A.
- BRISTOW, R. M., GILBERT, E. M., ABRAHAM, W. T., ADAMS, K. F., FOWLER, M. B., HERSHBERGER, R. E., KUBO, S. H., NARAHARA, K. A., INGERSOLL, H., YOUNG, S. & SHUSTERMAN, N. (1996). Carvedilol produces dose related improvements in left ventricular function and survival in subjects with chronic heart failure (MOCHA). Circulation 94, 2807–2816.
- BRODDE O. E. (1991). 1- 2-adrenoreceptors in human hearts: properties, functions, and alteration in chronic heart failure. Pharmacological reviews 43, 203–242.
- CALVERT, C. A., CHAPMAN, W. L., & TOAL, R. L. (1982). Congestive cardiomyopathy in Doberman Pinscher dogs. Journal American Veterinary Medical Association 181, 598–602.
- CALVERT, C. A., JACOBS, G. J., MEDLEAU, L. PICKUS, C. W., BROWN, J., MCDERMOTT, M. (1998). Thyroid-stimulating hormone tests in cardiomyopathic Doberman Pinschers: a retrospective study. Journal Veterinary Internal Medicine 12, 343–348.
- CIBIS INVESTIGATORS AND COMMITTEES (1994). A randomized trial of -blockade in heart failure: the Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 90, 1765–1773.
- CHASTIAN, C. B. & PANCIERA, D. L. (1995) Hypothyroid diseases. In Textbook of Veterinary Internal Medicine, 4th ed, pp 1487–1500. ed. S.J. Ettinger Philadelphia: W.B. Saunders.
- CHEN, H. H. & BURNETT J. C. Jr. (1998). C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. Journal of Cardiovascular Pharmacology 32, 3: S22–S28.
- CHEN, H. H. & BURNETT, J. C., Jr. (1999). The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proceedings of the Association of American Physicians 111, 406–416.
- CLARK, B.A., ELAHI, D. & FISH, L. (1991). Atrial natriuretic peptide suppresses osmostimulated vasopressin release in young and elderly humans. American Journal of Physiology 261, E252–E256.
- CLARK, W. A., RUDNICK, S. J., LAPTERS, J. J., ANDERSEN, L. C. & LAPOMTE, M. C. (1993). Regulation of hypertrophy and atrophy in cultured adult heart cells. Circulation Research 73, 1161–1176.
- CLERICO, A. & IERVASI, G. (1995). Alterations in metabolic clearance of atrial natriuretic peptides in heart failure: how do they relate to the resistance to atrial natriuretic peptides? Jornal of Cardiac Failure 1: 323–328.
- CODY, R. J. & LARAGH, J. H. (1988). The renin-angiotensinaldosterone system in chronic heart failure: pathophysiology and implications for treatment. In Drug treatment of heart failure, 2nd ed. ed. J.N. COHN, pp 79–104. Secaucus NJ: Advanced Therapeutics Communication International.
- CODY, R. J. (1997). The sympathetic nervous system and the renin-angiotensin-aldosterone system in cardiovascular disease. American Journal Cardiology 80, (9B): 9J–14J.
- COGAN, M. G. (1990a). Renal effects of atrial natriuretic factor. Annual Revue Physiology 52, 699–708.
- COGAN, M. G. (1990b). Atrial natriuretic peptide. Kidney International 37, 1148–1160.
- COHN, J. N., LEVINE, T. B., OLIVARI, M. T., GARBERG, V., LURA, D., FRANCIS, G. S. & SIMON, (1984). Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. The New England Journal of Medicine 311, 819–823.
- COLUCCI, W. S. & BRAUNWALD, E. (1997). Pathophysiology of heart failure. In Heart Disease: A Textbook of cardiovascular medicine 5th ed., ed. E. Braunwald, pp 394–420. Philadelphia: W.B. Saunders Co.
- COMMITTEE ON EVALUATION AND MANAGEMENT OF HEART FAILURE (1997). Guidelines for the evaluation and management of heart failure: report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal American College Cardiology 26, 1376–1398.
- THE CONSENSUS TRIAL STUDY GROUP (1987). Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The New England Journal of Medicine 316, 1429–1435.
- THE COVE STUDY GROUP (1995). Controlled clinical evaluation of enalapril in dogs with heart failure: results of Cooperative Veterinary Enalapril study group. Journal of Veterinary Internal Medicine 9, 243–252.
- DANILO, R. P., COHEN, I. S., BURKHOFF, D. & ROSEN, M. R. (1999). A role of the renin-angiotensin system in the evolution of cardiac memory. Journal of Cardiovascular Electrophysiology 10, 545–551.
- DELL’ITALIA, L. J., MENG, Q. C., BALCELLS, E., STRAETERKNOWLEN, I. M., HANKES, G. H., DILLON, R., CARTREE, R. E., ORR, R., BISHOP, S. P. & OPARIL, S. (1995). Increased ACE and chymase-like activity in cardiac tissue of dogs with chronic mitral regurgitation. American Journal of Physiology 269, H2065–H2073.
- THE DIGITALIS INVESTIGATION GROUP (1997). The effect of digoxin on mortality and morbidity in patients with heart failure. New England Journal Medicine 336, 525–531.
- DILLMAN, W. H. (1990). Biochemical basis of thyroid hormone action in the heart. American Journal of Medicine, 88, 626–630.
- DIXON, R. M., REID, S. W. J. & MOONEY, C. T. (1999). Epidemiological, clinical haematological and biochemical characteristics of canine hypothyroidism. Veterinary Record 145, 481–487.
- DZAU, V. J. & RE, R. (1994). Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation 89, 493–498.
- DZIMIRI, N. (1999). Regulation of -adrenoreceptor signaling in cardiac function and disease. Pharmacological reviews 51, 465–501.
- EICHORN, E. J. & STEVENSON, L. W. (1998). The ten most commonly asked questions about Beta-blockers for the heart failure. Cardiology in Review 6, 59–62.
- ETTINGER, S. J., BENITZ, A. M., ERICSSON, G. F., CIFELLI, S., JERNIGAN, A. D., LONGHOFER, S. L., TRIMBOLI., W. & HANSON, P. D. (1998). Effects of enalapril maleate on survival of dogs with naturally acquired heart failure. The Long-term Investigation of Veterinary Enalapril (LIVE) study group. Journal of American Veterinary Medical Association 213, 1573–1577.
- FELDMAN, A. M. (1993). Modulation of adrenergic receptors and G-transduction proteins in failing human ventricular myocardium. Circulation 87: IV27–34.
- FERGUSON, D. W. (1992). Digitalis and neurohormonal abnormalities in heart failure and implications for therapy. American Journal Cardiology 69, 24G–32G.
- FLORAS, J. S., HARA, K., WIGLE, E. D., DALY, P. & SENN, B. L. M. (1991). Sympathoneural profile of patients with idiopathic dilated cardiomyopathy (IDCM) (abstract). Canadian Journal of Cardiology 7, 104A.
- FLORAS, J. S. (1993). Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. Journal American College Cardiology 22 (supplement A): 72A–84A.
- FOULT, J.-M., TAVOLARO, O., ANTONY, I. & NITEMBERG, A. (1989). Coronary vasodilation induced by intracoronary enalaprilat: an argument for the role of a local renin-angiotensin-system in patient with dilated cardiomyopathy. European Heart Journal 10, 97–100.
- FOWLER, M. B. (1998). -blockers in heart failure. Do they improve the quality as well as the quantity of life? European Heart Journal 19, (Suppl. P), P17–P25.
- FRANCIS, G. S., BENEDICT, C., JOHNSTONE, D. E., KIRLIN, P. C., NICKLAS, J., LIANG, C. S., KUBO, S. H., RUBIN-TORETSKY, E. & YUSUF, S. (1990). Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure: a substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 82, 1724–1729.
- FRITSCHE, R., SCHELD, H. H. & MULCH, J. (1986). Effect of pimobendan on calcium sensitivity of skinned fibers isolated from human papillary muscles. British Journal of Pharmacology 89, 360–366.
- FUJINO, K., SPERELAKIS, N. & SOLARO, R. J. (1988). Sensitisation of dog and guinea pig heart myofilaments to Ca‡2 Activation and the inotropic effects of Pimobendan (Vetmedin): comparison with milrinone. Circulation Research 63, 911–922.
- FUKAMI, H., OKUNISHI, H. & MIYAZAKI, M. (1998). Chymase: its pathophysiological roles and inhibitors. Current Pharmapheceutical Design 4, 439–453.
- FUNAYA, H., KITAKAZE, M., NODE, K., MINAMINO, T., KOMAMURA, K. & HORI, M. (1997). Plasma adenosine levels increase in patients with chronic heart failure. Circulation 95, 1363–1365.
- GAVAGHAN, B. J. (2000). Is there still a role for digoxin in the treatment of cardiac disease? Australian Veterinary Journal 78, 528–529.
- GILBERT, W. M., SANDOVAL, A., LARABEE, P., RENLUND, D. G., O’CONNEL, J. B. & BRISTOW, M. R. (1993). Lisinopril lowers cardiac adrenergic drive and increases -receptor density in the failing human hearts. Circulation 88, 472–480.
- GINSBURG, R., ESSERMAN, L. J. & BRISTOW, M. R. (1983). Myocardial performance and extracellular ionized calcium in a severely failing human heart. Annales Internal Medicine 98, 603–606.
- GOETZ, K. L. (1988). Physiology and pathophysiology of atrial natriuretic peptides. American Journal Physiology 254, E1–E15
- GRANTHAM, J. A., BORGESON, D. D. & BURNETT, J. C. (1997) BNP: pathophysiological and potential therapeutic roles in acute congestive heart failure. American Journal of Physiology 272, R1077–1083.
- HAASS, M., DIETZ, R., FISHER, T. A., LANG, R. E. & KUTLER, W. (1988). Role of right and left atrial dimensions for release of atrial natriuretic peptide in left-sided valvular heart disease and idiopathic dilated cardiomyopathy. American Journal of Cardiology 62, 764–770.
- HA¨ GGSTRO¨ M, J, HANSSON, K., KVART, C., PEDERSEN, H. D., VOULTEENAHO, O. & OLSSON, K. (2000). Relationship between different natriuretic peptides and severity of naturally acquired mitral regurgitation in dogs with chronic myxomatous valve disease. Journal of Veterinary Cardiology 2, 7–16.
- HARE, J. M., BAUGMAN, K. L., KASS, D. A., GOODMAN, S. N. & LADENSON, P. W. (1991). Influence of dilated cardiomyopathy, myocarditis and cardiac transplantation on the relation between plasma atrial natriuretic factor and atrial pressures. American Journal Cardiology 67, 391–397.
- HASKING, G. J., ESLER, M. D., JENNINGS, G. L., BURTON, D., JOHNS, J. A. & KORNER, P. I. (1986). Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympatetic nervous activity. Circulation 73, 615–621.
- HAUSDORFF, W. P., CARON, M. G. & LEFKOWITZ, R. J. (1990). Turning off the signal: desensitization of -adrenergic receptor function. FASEB Journal 4, 2881–2898.
- HIRSCH, A. T., TALSNESS, C. E., SCHUNKERT, H., PAUL, M. & DZAU, V. J. (1991). Tissue-specific activation of cardiac angiotensin converting enzyme in experimental heart failure. Circulation Research 69, 475–482.
- The HOPE STUDY INVESTIGATORS (2000). Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355, 253–259.
- HUANG, H., ARNAL, J.-F., LLORENS-CORTES, C., CHALLAH, M., ALHENC-GELAS, F., CORVOL, P. & MICHEL, J. B. (1994). Discrepancy between plasma and lung angiotensinconverting enzyme activity in experimental congestive heart failure. A novel aspect of endothelium dysfunction. Circulation Research 75, 454–461.
- THE IMPROVE STUDY GROUP (1995). Acute and short term hemodynamic, echocardiographic and clinical effects of enalapril maleate in dogs with naturally acquired heart failure: results of the Invasive Multicentric Prospective Veterinary evaluation of Enalapril study group. Journal of Veterinary Internal Medicine 9, 234–252
- ITOH, H., NAKAO, K., SUGAWARA, A., SAITO, Y., MUKOYAMA, M., MORII, N., YAMADA, T., SHIONO, S., ARAI, H. & HOSODA, K. (1988). Gamma-atrial natriuretic polypeptide-derived peptides in human plasma: cosecretion of N-terminal gamma-ANP fragment and alpha-ANP. Journal of Clinical Endocrinology Metabolism 67, 429–437.
- JAMALL, I. N., PAGEL, P. S., HETTRICK, D. A., LOWE, D., KERSTEN, J. R., TESSMER, J. P. & WARLIER, D. C. (1997). Positive inotropic and lusitropic effects of triiodothyronine in conscious dogs with pacing-induced cardiomyopathy. Anesthesiology 87, 102–109.
- JESSUP, M. & BROZENA, S. (2000). Treatment of advanced heart failure. Cardiology in Review 3, 148–157.
- KAWAI, M., NARUSE, M., YOSHIMOTO, T., NARUSE, K., SHIONOYA, K., TANAKA, M., MORISHITA, Y., MATSUDA, Y., DEMUA, R. & DEMURA, H. (1996). C-type natriuretic peptide as a possible local modulator of aldosterone secretion in bovine adrenal zona glomerulosa. Endocrinology 137, 42–46.
- KEISTER, D. M. (1988). A summary of phase IV clinical data on milrinone (Wincardin), a canine cardiotonic: protocol H, New York, Sterling-Winthrop.
- KITTLESON, M. D. (1994). Left ventricular function and failure. Part II. The Compendium on Continuing Education for Practicing Veterinarian 16, 1001–1017.
- KITTLESON, M. D., EYESTER, G. E., KNOWLEN, G. G., OLIVIER, N. B. & ANDERSON, L. K. (1985). Efficacy of digoxin administration in dogs with idiopathic congestive cardiomyopathy. Journal American Veterinary Medical Association 186, 162–165.
- KITTLESON, M. D., PIPERS, F. S., KNAUER, K. W., KEISTER, D. M., KNOWLEN, G. G. & MINER, W. S. (1985). Echocardiographic and clinical effects of milrinone in dogs with myocardial failure. American Journal Veterinary Research 46, 1659–1664.
- KITTLESON, M. D. & KIENLE, R. D. (1998). Management of heart failure. In Small Animal Cardiovascular Medicine eds. M.D. Kittleson & R.D. Kienle, pp 149–189, St. Louis: Mosby Inc.
- KLEEMANN, R., LE BOBINNEC, G., BRUYERE, D., JUSTUS, C. & SCHMIDT, H. (1998). Clinical efficacy of Vetmedin in comparison to digoxin for the treatment of congestive heart failure in dogs. Proceedings of the 4th European FECAVA SCIVAC Congress, Bologna 18th–21th June 513.
- KLUGER, J., CODY, R. J. & LARAGH, J. H. (1982). The contribution of sympathetic tone and the renin-angiotensin-aldosterone system in severe chronic heart failure: response to specific inhibitors (prazosin and captopril). American Journal Cardiology 49, 1667–1674.
- KOCH, J., PEDERSEN, H. D., JENSEN, A. L., FLAGSTAD, A. & POULSEN, K. (1995). Activation of the renin-angiotensin system in dogs with asymptomatic and symptomatic dilated cardiomyopathy. Research Veterinary Science 59, 172–175.
- KUBO, S., H., GOLLUB, S., BOURGE, R., RAHKO, P., COBB, F., JESSUP, M., BROZENA, S., BRODSKY, M., KIRLIN, P. & SHANES, J. (1992). Beneficial effects of pimobendan on exercise tolerance and quality of life in patients with heart failure. Results of a multicenter trial. The Pimobendan Multicenter Research Group. Circulation 85, 942–949.
- LANDZBERG, J. S., PARKER, J. D., GAUTHIER, D. F. & COLUCCI, W. S. (1991). Effects of myocardial alpha1-adrenergic receptor stimulation and blockade on contractility in humans. Circulation 84, 1608–1614.
- LANG, C., CHOY, A. & STRUTHERS, D. (1992) Atrial and brain natriuretic peptides: a dual natriuretic peptide system involved in circulatory homeostasis. Clinical Science 83, 519–527.
- LEIMBACH, W. N. Jr., WALLIN, G., VICTOR, R. G., AYLWARD, P. E., SUNDLOF, G. & MARK, A. L. (1986). Direct evidence from intrarenal recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73, 913–919.
- LIANG, C. S., FAN, T. H. M., SULLEBARGER, J. T. & SAKAMOTO, S. (1989). Decreased adrenergic neuronal uptake activity in experimental right heart failure. A chamber-specific contributor to beta-adrenoceptor downregulation. Journal of Clinical Investigation 84, 1267–1275.
- LOMBARD, C. W. (2000). Therapy of congestive heart failure in dogs with pimobendan. Proceedings of 17th American College of Veterinary Internal Medicine Forum, May 25–28, 107–109.
- LUBSEN, J., JUST, H., HJALMARSSON, A. C., LA FRAMBOISE, D., REMME, W. J., HEINRICH-NOLS, J., DUMONT, J. M. & SEED, P. (1996). Effect of pimobendan on exercise capacity in patients with heart failure: main results from the Pimobendan in Congestive Heart Failure (PICO) trial. Heart, 76, 223–231.
- LUMBERS, E. R. (1999). Angiotensin and aldosterone. Regulatory Peptides 80, 91–100.
- LUIS FUENTES, V., KLEEMANN, R., JUSTUS, C., FRENCH, A., SCHOBER, K.E. & CORCORAN, B. M. (1998). The effect of the novel inodilator Pimobendan (Vetmedin) on heart failure status in Cocker Spaniels and Dobermans with idiopathic dilated cardiomyopathy. 41st British Small Animal Veterinary Congress, Birmingham 2nd–5nd April, 284.
- MACDONALD, P. S., KEOGH, A. M., ABOYOUN, C. L., LUND, M., AMOR, R. & MCAFFREY, D. J. (1999). Tolerability and efficacy of carvedilol in patients with New York Heart Association class IV heart failure. Journal American College Cardiology 33, 924–931.
- MALHOTRA, R., SADOSHIMA, J., BROSIUS, F.C. 3rd, & IZUMO S. (1999). Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circulation Research 85, 137–146.
- MALIK, F. S., LAVIE, C. J., MEHRA, M. R.,. MILANI, R. V. & RE, R. N. (1997). Renin-angiotensin system: Genes to bedside. American Heart Journal 134, 514–526.
- MANN, D. L., KENT, R. L., PARSONS, B. & COOPER, G. (1992). Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85, 790–804.
- MASON, D. T., ZELIA, R. & AMSTERDAM, E. A. (1972). Unified concept of the mechanism of action of digitalis: influence of ventricular function and cardiac disease on hemodynamic response to fundamental contractile effect. In Basic and Clinical Pharmacology of Digitalis, eds. B.H. Marks & A.M. Weissler. St. Louis: Mosby Inc.
- MORUZZI, P., DORIA, E. & AGOSTINI, P. G. (1996). Mediumterm effectiveness of L-thyroxine treatment in idiopathic dilated cardiomyopathy. American Journal of Medicine 101, 461–467.
- NEWTON, G. E. & PARKER, J. D. (1996). Acute effects of 1- selective and nonselective 1-adrenergic receptor blockade on cardiac sympathetic activity in congestive heart failure. Circulation 94, 353–358.
- NICHOLLS, D. P., ONUOHA, G. N., McDOWELL, G., ELBORN, J. S., RILEY, M. S., NUGENT, A. M., STEELE, I. C., SHAW, C. & BUCHANAN, K. D. (1996). Neuroendocrine changes in chronic cardiac failure. Basic Research Cardiology 91, 13–20
- O’GRADY, M. R., HOME, R. & GORDON, S. G. (1997). Does angiotensin converting enzyme inhibitor therapy delay the onset of congestive heart failure or sudden death in Doberman Pinchers with occult dilated cardiomyopathy? Journal of Veterinary Internal Medicine (abstract) 11, 138.
- OHTANI, S., FUJIWARA, H., HASEGAWA, K., DOYAMA, K., INADA, T., TANAKA, M., FUJIWARA, T. & SASAYAMA, S. (1997). Upregulated expression of angiotensin II type 1 receptor gene in human pathologic hearts. Journal of Cardiol Failure 3, 303–310.
- PACKER, M. (1987a). Adaptive and maladaptive actions of angiotensin II in patients with severe congestive heart failure. American Journal of Kidney Disease 10, 66–73.
- PACKER, M., LEE, W. H., KESSLER, P.D., GOTTLIEB, S. S., BERNSTEIN, J. L. & KUKIN, M. L. (1987b). Role of neurohormonal mechanisms in determing survival in patients with severe chronic heart failure. Circulation 75, 80–92.
- PACKER, M. D., GHEORGHIADE, M., YOUNG, J. B., CONSTANTINI, P. J., ADAMS, K. F., CODY, R. J., SMITH, L. K., VAN VOORHEES, L., GOURLEY, L. A. & JOLLY, M. K. (1993). Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE study. New England Journal of medicine 329, 1–7.
- PACKER, M. D., BRISTOW, M. R., COHN, J. N., COLUCCI, W. S., FOWLER, M. B., GILBERT, E. M. & SHUSTERMAN, N. H. FOR THE U.S. CARVEDILOL HEART FAILURE STUDY GROUP (1996). The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. New England Journal of Medicine 334, 1349–1355.
- PACKER, M. (1997). Effects of beta-adrenergic blockade on survival of patients with chronic heart failure. American Journal of Cardiology, 80 (Suppl. A), 46L–54L.
- PACKER, M., CARVER, J. R., RODEHEFFER, R. J., IVANHOE, R. J., DIBIANCO, R., ZELDIS, S. M., HENDRIX, G. H., BOMMER, W. J., ELKAYAM, U. & KUKIN, M. L. (1991). Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. New England Journal Medicine 325, 1468–1478.
- PANCIERA, D. L. (1994). An echocardiographic and electrocardiographic study of cardiovascular function in hypothyroid dogs. Journal of American Veterinary Medicine Association 205, 996–999.
- PANCIERA, D. L. (1999). Is it possible to diagnose canine hypothyroidism? Journal of Small Animal Practice 40, 152–157.
- PEDERSEN, H. D., OLSEN, L. H. & ARNORSDOTTIR, H. (1995a). Breed differences in the plasma renin activity and plasma aldosterone concentration of dogs. Journal Veterinary Medicine Series A 42, 435–441.
- PEDERSEN, H. D., KOCH, J., POULSEN, K., JENSEN, A. L. & FLAGSTAD, A. (1995b). Activation of the renin-angiotensin system in dogs with asymptomatic and mildly symptomatic mitral valvular insufficiency. Journal of Veterinary Internal Medicine 9, 328–331.
- POULEUR, H., GURNE’, O., HANET, C., BALASIM, H., VAN MECHELEN, H. & CHARLIER, A. A. (1988). Effects of pimobendan (UD-CG 115) on the contractile function of the normal and ‘‘postischemic’’ canine myocardium. Journal of Cardiovascular Pharmacology 11, 100–106.
- RE, G., BADINO, P., BERGAMASCO, L. BORGARELLI, M., ODORE, R. & TARDUCCI, A. (1997). Down regulation of lymphocyte adrenoreceptor subtypes in dogs affected by dilated cardiomyopathy. Journal of Veterinary Pharmacology and Therapeutics 20, (Suppl. 1): 236–237.
- RE, G., BERGAMASCO, L., BADINO, P., BORGARELLI, M., ODORE, R., TARDUCCI, A. & ZANATTA, R. (1999). Canine dilated cardiomyopathy: lymphocyte and cardiac 1 and - adrenoceptor concentrations in normal and affected Great Danes. The Veterinary Journal 158, 120–127.
- REMME, W. J. (1993). Inodilator therapy for heart failure. Early, late, or not at all? Circulation 87, IV97–107.
- ROSE, C. P., BURGESS, J. H. & COUSINEAU, D. (1985). Tracer norepinephrine kinetics in coronary circulation of patient with heart failure secondary to chronic pressure and volume overload. Journal of Clinical Investigation 76, 1740–1747.
- RUTHERFORD, J. P., VATNER, S. F. & BRAUNWALD, E. (1979). Adrenergic control of myocardial contractility in consious dogs. American Journal of Physiolology 237, H590– H596.
- SABBAH, H. N., SHIMOYAMA, H., KONO, T., GUPTA, R. C., SHAROV, V. G., SCICLI, G., LEVINE, T. B. & GOLDESTEIN, S. (1994). Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilatation in dogs with reduced ejection fraction. Circulation 89, 2852– 2859.
- SAITO, Y., NAKAO, K., ARAI, H., SUGAWARA, A., MORII, N., YAMADA, T., ITOH, H., SHIONO, S., MUKOYAMA, M. & OBATA, K. (1987). Atrial natriuretic polypeptide (ANP) in human ventricle. Increased gene expression of ANP in dilated cardiomyopathy. Biochemical Biophysiology Research Communication 148, 211–217.
- SHAHRARA, S., TIDHOLM, A., DRVOTA, V., HA¨ GGSTRO¨ M, J. & SYLVEN, C. (1999). Upregulation of thyroid hormone receptor mRNA beta-1 and beta-2 subtypes in the myocardium of dogs with naturally occurring dilated cardiomyopathy or chronic valvular disease. American Journal of Veterinary Research 60, 848–852.
- SIMPSON, P. & McGRATH, A. (1983). Norepinephrine-stimulated hypertrophy of coltured rat myocardial cells in a alpha adrenergic response. Journal of Clinical Investigation 72, 732–738.
- SISSON, D. D. & THOMAS, W. P. (1995). Myocardial disease. In Textbook of Veterinary Internal Medicine 4th ed, ed. S.J. Ettinger, pp 995–1005. Philadelphia: W.B. Saunders Co.
- SISSON, D. D. (1999). Biochemical detection of left ventricular dysfunction in dogs. Proceedings of 17th American College of Veterinary Internal Medicine Forum 10–13 June, Chicago, 68.
- SISSON, D. D., THOMAS, W. P. & KEENE, B. W. (2000). Primary myocardial disease in the dog. In Textbook of Veterinary Internal Medicine 5th Edn.: S.J. Ettinger & E.C., Feldman, pp 874–895. Philadelphia: W.B. Saunders Co.
- SLATTON, M. L., IRANI, W. L., HALL, S. A., MARCOUX, L. G., PAGE, R. L., GRAYBURN, P. A. & EICHHORN, E. J. (1997). Does digoxin provide additional hemodynamic and autonomic benefit at higher doses in patients with mild to moderate heart failure and normal sinus rhythm? Journal American College Cardiology 29, 1206–1213.
- THE SOLVD INVESTIGATORS (1991). Effect of enalapril on survival in patients with reduced left ventricular ejection fractions. The New England Journal of Medicine 325, 293–302
- The SOLVD INVESTIGATORS (1992). Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The New England Journal of Medicine 327, 685–691
- STRUTHERS, A. D. (1994). Ten years of natriuretic peptide research: a new dawn for their diagnostic and therapeutic use? British Medicine Journal 308, 1615– 1619.
- TAKAHASHI, N., CALDERONE, A., IZZO, N. J., Jr., MAKI, T. M., MARSH, J. D. & COLUCCI, W. S. (1994). Hypertrophic stimuli induce transforming growth factor-beta1 expression in rat ventricular myocytes. Journal of Clinical Investigation 94, 1470–1476.
- TAN, L. J., JALIL, E., PICK, R., JANICKI, J. S. & WEBER, K. T. (1991). Cardiac myocyte necrosis induced by angiotensin II. Circulation research 69, 1185–1195.
- TARDUCCI, A., BORGARELLI, M., RE, G., BERGAMASCO, L., BADINO, P., ODORE, R. & BUSSADORI, C. (1997). Lymphocyte beta adrenoceptor in dogs with dilated cardiomyopathy (abstract). Proceedings of 7th Annual Congress of the European Society of Veterinary Internal Medicine 12–14 September, Lyon (France). 194 THE VETERINARY JOURNAL, 162, 3
- THIBAULT, G., AMIRI, F. & GARCIA, R. (1999). Regulation of natriuretic peptide secretion by the heart. Annual Review of Physiology 61, 193–217.
- THOMAS, J. A. & MARKS, B. H. (1978). Plasma norepinephrine in congestive heart failure. American Journal of Cardiology 41, 233–243.
- TIDHOLM, A., HAGGSTROM, J., BORGARELLI, M. & TARDUCCI, A. (2001). Camine idiopathic dilated cardiomyopathy. Part 1: Aetiology, clinical characteristics, epidemiology and pathology. The Veterinary Journal.
- TIDHOLM, A., HAGGSTROM, J. & HANSSON, K. (2000). Effects of naturally occuring symptomatic and asymptomatic dilated cardiomyopathy on the renin-angiotensinaldosterone system, atrial natriuretic peptide and thyroid hormone concentrations in dogs. In Canine Idiopathic Dilated Cardiomyopathy: Epidemiology Histopatology and Pathophysiology, ed. A. Tidholm, Doctoral Thesis, Swedish University of Agricultural Sciences, Uppsala 2000 paper VI.
- TREADWAY, G. (1985). Clinical safety of intravenous amrinone. American Journal of Cardiology 56, 39B–40B.
- TSUTSUI, H., SPINALE, F. G., NAGATSU, M., SCHMID, P. G., ISHIHARA, K., DE FREYTE, G., COOPER, G. 4th & CARABELLO, B. A. (1994). Effects of chronic beta-adrenergic blockade on the left ventricular and cardiocyte abnormalities of chronic canine mitral regurgitation, Journal Clinical Investigation 93, 2639–2648.
- UNGER, T., GANTEN, D. & LANG, R. E. (1987). Effects of converting enzyme inhibitors on tissue converting enzyme and angiotensin II: therapeutic implications. American Journal of Cardiology 59, 18D–22D.
- UNGER, T. (2000). Neurohormonal modulation in cardiovascular disease. American Heart Journal 139, S2–S8.
- URETSKY, B. F., YOUNG, J. B., SHAHIDI, F. E., YELLEN, L. G., HARRISON, M. C. & JOLLY, M. K.(1993). Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. Journal American College Cardiology 22, 955–962.
- VAN MEEL, J. C. A. & DIEDEREN, W. (1989). Hemodynamic profile of the cardiotonic agent pimobendan. Journal of Cardiovascular Pharmacology 14, (suppl 2), S1–S6.
- VATNER, D. E., VATNER, S. F., FUJII, A. N. & HOMCY, G. J. (1985). Loss of high affinity cardiac receptors in dogs with heart failure. Journal of Clinical Investigation 76, 2259–2264.
- VIQUERAT, C. E., DALY, P. A., SWEDBERG, K., CURRAN, D., PARMELEY, W. W. & CHATTERJEE, K. (1985). Endogenous cathecolamine levels in chronic heart failure. American Journal of Medicine 78, 455–459.
- VOLLMAR, A. M., REUSCH, C., KRAFT, W. & SCHULZ, R. (1991). Atrial natriuretic peptide concentration in congestive heart failure, chronic renal failure and hyperadrenocorticism. American Journal of Veterinary Research 52, 1831–1834.
- WAAGSTEIN F., CAIDHAL, K., WALLENTIN, I., BERGH, C.-H. & HJALMARSON, A. (1989). Long term -blockade in dilated cardiomyopathy. Effects of short- and long-term metoprolol treatment followed by withdrawal and readministration of metoprolol. Circulation 80, 551–563.
- WAAGSTEIN, F., BRISTOW, M. R., SWEDBERG, K., CAMERINI, F., FOWLER, M. B., SILVER, M. A., GILBERT, E. M., JOHNSON, M. R., GROSS, F. G. & HJALMARSON, A. (1993). Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Lancet 342, 1441–1446.
- WALD, H., SCHERZER, P. & POPOVTZER, M. M. (1991). Na,KATPase in isolated nephron segments in rats with experimental heart failure. Circulatory Research 68, 1051–1058.
- WARE, W. A., LUND, D. D., SUBIETA, R. R. & SCHMID, P. G. (1990). Sympathetic activation in dogs with congestive heart failure caused by chronic mitral valve disease. Journal of American Veterinary Medical Association 197, 1475–1481.
- WEI, C., HEUBLINE, D. & BURNETT, J. (1991). Pathophysiologic concentrations of human brain natriuretic peptide have functionally important biological actions in vivo. Journal of the American Society of Nephrology 2, 422–425.
- WEI C. M., HEUBLEIN, D. M., PERELLA, M. A., Lerman, A. RODEHEFFER, R. J., MCGREGOR, C. G., EDWARDS, W. D., SCHAFF, H. V. & BURNETT, J. C., Jr. (1993). Natriuretic peptide system in human heart failure. Circulation 88, 1004–1009.
- WILKE, A., FUNCK, R., RUPP, H. & BRILLA, C. G. (1996). Effects of the renin-angiotensin-aldosterone system on the cardiac interstitium in heart failure. Basic Research in Cardiology 91, 79–84.
- WYNNE, J. & BRAUNWALD, E. (1997). The cardiomyopathies and myocardites. In Heart Disease: A Textbook of Cardiovascular Medicine, 5th ed., ed. E. Braunwald, pp 1404– 1453. Philadelphia: W.B. Saunders Co.
- YAMADA, S., OKHURA, T., UCHIDA, S., INABE, K., IWATANI, Y., KIMURA, R., HOSHINO, T. & KABURAGI, T. (1996). A sustained increase in -adrenoreceptors during long term therapy with metoprolol and bisoprolol in patients with heart failure from idiopathic dilated cardiomyopathy. Life Science 58, 1737–1744
- YASHUE, H., OBATA, K., OKUMURA, K., KUROSE, M., OGAWA, H., MATSUYAMA, K., JOUGASAKI, M., SAITO, Y., NAKAO, K. & IMURA, H. (1989). Increased secretion of atrial natriuretic polypeptide from the left ventricle in patients with dilated cardiomyopathy. Journal Clinical Investigation 83, 46–51
- YOSHIMURA, M., YASUE, H., OKUMURA, K., OGAWA, H., JOUGASAKI, M., MUKOYAMA, M., NAKAO, K. & IMURA, H. (1993). Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 87, 464–469
^Наверх









