An echocardiographic study of healthy Border Collies with normal reference ranges for the breed
Author information
Jacobson J.H., Boon J.A., Bright J.M. An echocardiographic study of healthy Border Collies with normal reference ranges for the breed // J Vet Cardiol. 2013 Jun;15(2):123-30.
Abstract
OBJECTIVES: The objectives of this study were to obtain standard echocardiographic measurements from healthy Border Collies and to compare these measurements to those previously reported for a general population of dogs.
ANIMALS: Standard echocardiographic data were obtained from twenty apparently healthy Border Collie dogs. These data (n = 20) were compared to data obtained from a general population of healthy dogs (n = 69).
METHODS: Border Collies were deemed healthy based on normal history, physical examination, complete blood count, serum biochemical profile, electrocardiogram, and blood pressure, with no evidence of congenital or acquired heart disease on echocardiographic examination. Standard two dimensional, M-mode, and Doppler echocardiographic measurements were obtained and normal ranges determined. The data were compared to data previously obtained at our hospital from a general population of normal dogs.
RESULTS: Two dimensional, M-mode, and Doppler reference ranges for healthy Border Collies are presented in tabular form. Comparison of the weight adjusted M-mode echocardiographic means from Border Collies to those from the general population of dogs showed Border Collies to have larger left ventricular systolic and diastolic dimensions, smaller interventricular septal thickness, and lower fractional shortening.
CONCLUSIONS: There are differences in some echocardiographic parameters between healthy Border Collies and the general dog population, and the echocardiographic reference ranges provided in this study should be used as breed specific reference values for Border Collies.
Abbreviations
- 2D two dimensional
- Ao aortic root diameter
- CW continuous wave
- d diastole
- EPSS E-point-to-septal separation IVRT isovolumic relaxation time IVS interventricular septal thickness LA left atrial dimension
- LIMP left ventricular index of myocardial performance LV left ventricular
- LVET left ventricular ejection time
- LVID left ventricular internal dimension
- LVPEP left ventricular pre-ejection period LVW left ventricular wall thickness MCO mitral closure to opening PV pulmonary valve
- PW pulsed wave
- s systole
Introduction
Although echocardiographic values for the general dog population have been published, it is now known that there can be variation based on breed, body size, somatotype and body surface area.1-13 Echocardiographic reference ranges derived from some dog breeds may be misleading for others, limiting their clinical usefulness. It has been shown that parameters of chamber size, wall thickness and systolic function are different in breeds known for their athletic ability.4,10,14,15 Border Collies are herding dogs recognized as extremely energetic, athletic, and acrobatic. Healthy Border Collies are, therefore, likely to have cardiac structural and functional measurements different than those of the general dog population. Yet normal echocardiographic values for this breed are currently unavailable. Breed specific echocardiographic reference ranges may be needed for evaluation of Border Collies suspected of having heart disease in order to make accurate cardiac diagnoses and to assess disease severity. The objectives of this study were to obtain standard echocardiographic measurements from healthy Border Collies and to compare these measurements to those previously reported for a general population of dogs.
Animals, materials and methods
Animals
For this study, 23 clinically healthy Border Collie dogs were evaluated prospectively at the James L. Voss Veterinary Teaching Hospital at Colorado State University from 2010 to 2011. This study was approved by the Colorado State University Institutional Animal Care and Use Committee, and informed consent was provided by all owners. Inclusion criteria were normal history, physical examination, clinicopathologic assessment, electrocardiogram, and blood pressure with no evidence of congenital or acquired heart disease on standard echocardiographic examination. Clin- icopathologic assessment included a complete blood count and biochemistry profile.
Echocardiographic data obtained from the Border Collies were compared to previously published data from a general population of dogs studied in our hospital.16 These dogs were also clinically healthy based on physical examination and echo- cardiographic study. There were 69 dogs in this group, including 54 purebred dogs representing 18 different breeds and an additional 15 mixed-breed dogs. These dogs ranged from 1 to 12 years old (mean, 4 years) with body weight from 3.9 to 97.7 kg (mean 28 kg).
Materials and methods
Echocardiographic examinations consisting of two dimensional (2D), M-mode, color Doppler and spectral Doppler assessments were performed with a GE Vivid 7 echocardiographic recorder3 using a GE 5S, phased array, multifrequency transducer13 and coded harmonic imaging in all Border Collies. A GE Vivid 5 echocardiographic recorderc with phased array transducers ranging in frequency from 2.5 to 7.5 MHz was used in the previous study of non-Border Collies.16 All dogs (Border Collies and non-Border Collies) had echocardiographic data obtained in a dark, quiet room while unsedated and loosely restrained in both right and left lateral recumbency on an echocardiographic table with a cutout for scanning from below the animal.
Measurements included 2D aortic root diameter (Ao) and left atrial dimension (LA) measured from the right parasternal short-axis image of the heart base.17 From the right parasternal short-axis 2D image at the chordae level, interventricular septal thickness (IVS), left ventricular internal dimension (LVID), and left ventricular wall thickness (LVW) in diastole (d) and systole (s) were obtained. These same measurements as well as E-point-to-septal separation (EPSS) were also obtained using M-mode images obtained from the right parasternal long- axis inflow outflow view.
a Vivid 7, GE Healthcare, Waukesha, WI, USA. b GE Ultrasound 5S 2.25-4.75 MHz, GE Healthcare, Waukesha, WI, USA.
c Vivid 5, GE Healthcare, Waukesha, WI, USA.
All standard 2D and M- mode variables, including fractional shortening, were measured according to recommendations set by the American Society of Echocardiography and published methodology in the veterinary liter- ature.18-20 Two-dimensional measurement of the pulmonary valve (PV) annulus was obtained at the level of the annulus immediately prior to valve opening using right parasternal transverse images.21 Left ventricular (LV) diastolic length was measured from the apex to the mitral valve annulus using left apical four chamber views. The LV sphericity index was calculated by dividing LV length by the LVIDd obtained from M-mode images.22 Peak aortic blood flow velocity and iso- volumic relaxation time (IVRT) were obtained from the left apical 5-chamber view using continuous wave (CW) Doppler. Peak aortic blood flow velocity was measured as the maximal velocity on the CW recordings of aortic blood flow, IVRT was measured as the time from the cessation of LV outflow (determined by identifying the Doppler click associated with closure of the aortic valve) to the onset of LV inflow, LV ejection time (LVET) was measured as the time from onset to cessation of the aortic blood flow signal, and LV pre-ejection period (LVPEP) was measured from the start of the QRS complex to the onset of aortic blood flow.23-25
Blood flow velocities in the pulmonary artery were recorded with pulsed wave (PW) Doppler from a right parasternal modified long axis view with the sample gate placed within the artery just distal to the pulmonary valve.26-28 The peak blood flow velocity was measured from the PW recordings as the maximal value of the systolic flow signal. LV inflow velocities were obtained with PW Doppler using the left parasternal apical four-chamber view placing the sample gate in the ventricle at a depth corresponding to the tips of the mitral valve leaflets when wide open.23,24 Mitral closure to opening (MCO) was measured as the interval between the end of transmitralflow (end of A wave) and onset of LV inflow (start of E wave). LV index of myocardial performance (LIMP), also referred to as the Tei index or myocardial performance index (MPI), was calculated using the LVET and MCO in the following equation: (MCO - LVET)/LVET.29 No angle corrections were needed, as parallel alignment of the Doppler cursor was possible in all dogs.
All cardiac valves and the interatrial and interventricular septae were interrogated using color Doppler. Morphology of all four valves was evaluated with 2D imaging in each imaging plane that showed the valve. For color Doppler evaluation, care was taken to use a consistent pulse repetition frequency of 70-90 c/s with gain set just below the color speckling artifact. Regurgitations through each of the four valves were quantified with color Doppler as trace, mild, moderate, or severe using the regurgitant jet area method for the atrioventricular valves, the jet width method for the aortic valve, and the jet length method for the pulmonary valve.30-33 For all valves, physiologically normal, trace to mild valvular regurgitation was defined as visible color remaining in close proximity to the valve with little extension into the receiving chamber and of short duration (in man <100 ms). Furthermore, physiological trace and mild regurgitations showed no retrograde flow acceleration, had low pressure gradients which did not come close to estimating normal pressure differentials when attempted, and generated no murmurs.28,30,34-36
Mild mitral or tricuspid valve regurgitations which were not considered physiologic were defined as color jets not meeting the criteria for trace and physiologically mild jets, and occupying up to 20% of the atrial chamber. Moderate mitral or tricuspid valve regurgitations were defined as a color jet occupying 20-50% of the atrial chamber. Finally, severe mitral or tricuspid regurgitations were defined as jets exceeding 50% of the atrial chamber. All dogs with pathologically mild, moderate, or severe mitral or tricuspid regurgitation were excluded from the study.31-33
For all M-mode measurements, 2D measurements and Doppler time intervals, a lead II electrocardiogram was recorded simultaneously, and three representative cardiac cycles were measured and averaged. Heart rates were the instantaneous rates obtained from the M-mode sweep from which measurements were obtained.
Indirect arterial blood pressure was measured using Doppler sphygmomanometryd with dogs in left lateral recumbency using the right dorsal metatarsal artery. The mean of 3 systolic pressure measurements was recorded. Dogs with a systolic blood pressure <90 or >160 mmHg were excluded from the study.
For each echocardiographic response variable, a Shapiro-Wilk test was used to determine that data were normally distributed. Mean and standard deviation with 95% confidence intervals were determined for all continuous response variables. A comparison of the weight adjusted means for M-mode echocardiographic response variables was made using a one-way analysis of covariance (weight was the covariate) with Border Collies and the general population of dogs as groups.e In the Border Collie group, the relationship between LVIDd and heart rate was evaluated using simple linear regression and Pearson correlation coefficients.
d Ultrasonic Doppler Flow Detector, Parks Medical Electronics Inc, Aloha, OR, USA.
In the Border Collie group, the relationship between weight and continuous echocardiographic variables was also evaluated with simple linear regression and Pearson correlation coefficients. Relationships and group differences were considered significant when P < 0.05.
Results
Twenty Border Collies met the criteria for inclusion in the current study. Three dogs were excluded for the following reasons: presence of an atrial septal defect observed on color Doppler interrogation of the interatrial septum; presence of moderate mitral regurgitation; and inability to obtain accurate measurements due to poor echocardiographic imaging quality during the exam. The Border Collie group consisted of 9 neutered males and 11 spayed females. The dogs in this group ranged in age from 2 to 12 years (mean, 6 years). The study represented a range of sizes from 15 kg to 29 kg (mean, 19 kg).
Heart rate and Doppler blood pressure measurements were obtained in all 20 Border Collies. Heart rate ranged from 54 bpm to 159 bpm (mean, 85 bpm) and systolic blood pressure ranged from 90 mmHg to 155 mmHg (mean, 125 mmHg). All of the 20 dogs included in the study had normal appearing valve morphology; however, physiologic regurgitations were frequently noted; 8/20 (40%) had trace mitral regurgitation; 1/20 (5%) had mild, physiologic mitral regurgitation; 12/20 (60%) had trace tricuspid regurgitation; 1/20 (5%) had mild, physiologic tricuspid regurgitation; and 13/20 (65%) had trace pulmonary valve insufficiency. No dog had aortic regurgitation. Dogs with trace to mild regurgitations that met the criteria for normal physiological retrograde flow as previously described, were not excluded.
The two dimensional, M-mode, and Doppler echocardiographic reference ranges for healthy Border Collies are shown in Tables 1 through 3 respectively. (The raw data are included as a Supplemental Data File On Line.)
e SAS® Institute Inc., Version 9.3, Cary, NC, USA.
Table 1 Two dimensional (2D) measurements in 20 Border Collies.
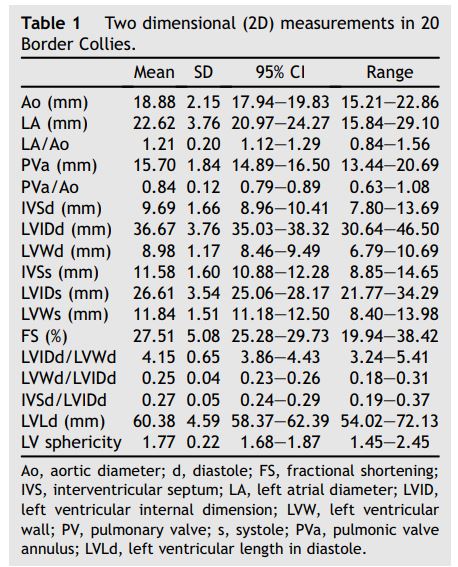
No significant differences were observed between males and females. Heart rate did not correlate significantly with LVIDd. Body weight correlated significantly but weakly with LV length (P = 0.0002, r = 0.73), LVWd (P = 0.01, r = 0.5), and LVWs (P = 0.03, r = 0.49).
Table 2 M-mode Collies. measurements in 20 Border
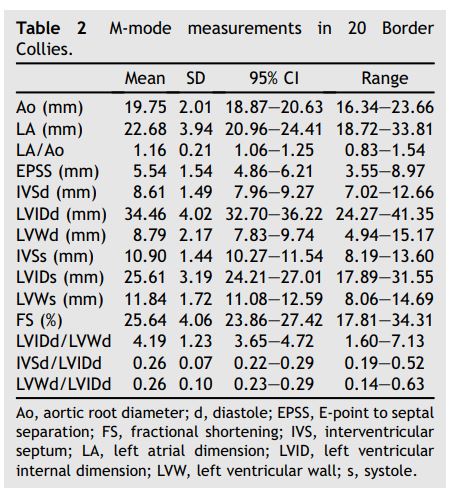
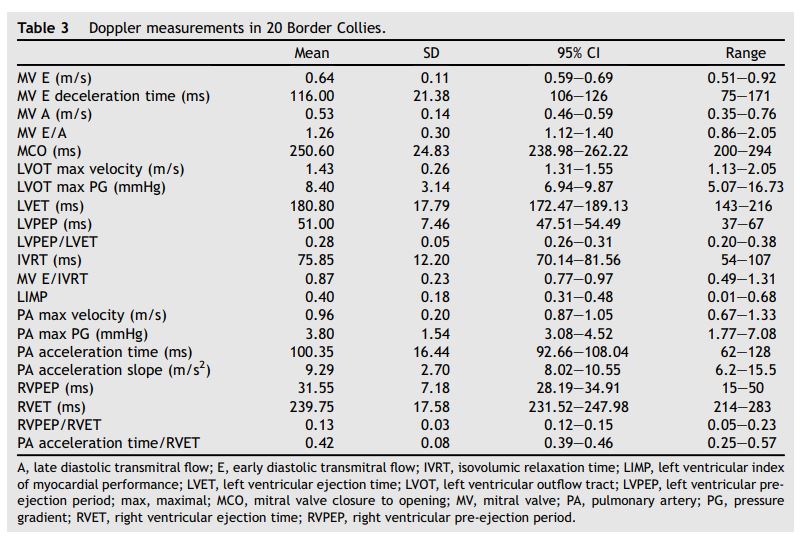
Table 3 Doppler measurements in 20 Border Collies
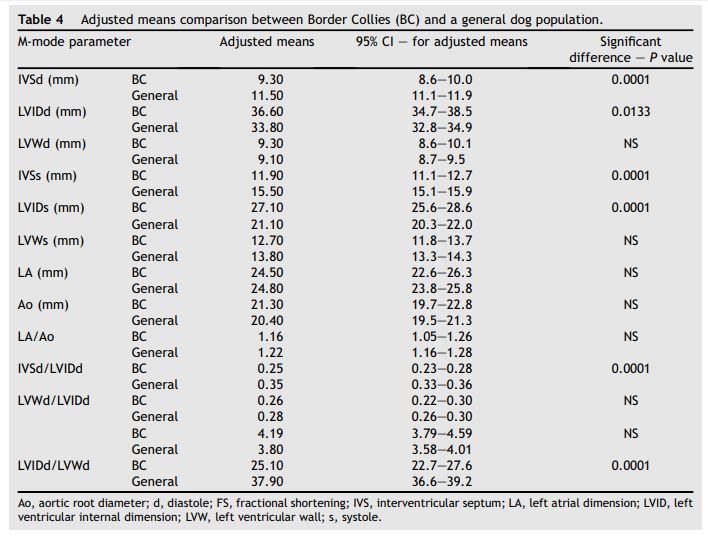
Weight adjusted M-mode echocardiographic means for Border Collies and the general population of dogs are shown in Table 4. Parameters that were significantly less in the Border Collie group than in the general population included: IVSd and IVSs (9.3 mm vs. 11.5 mm; P = 0.0001 and 11.9 mm vs. 15.5 mm; P = 0.001, respectively), IVSd/LVIDd ratio (0.25 vs. 0.35; P = 0.0001), and fractional shortening (FS, 25.1% vs. 37.9%; P = 0.0001). Parameters that were significantly greater in the Border Collies compared to the general population included LVIDd and LVIDs (36.6 mm vs. 33.8 mm; P = 0.01 and 27.1 mm vs. 21.1 mm; P = 0.0001, respectively).
Discussion
There are over 37 papers investigating breed specific and generic echocardiographic parameters.26 These studies add support to the belief that breed variation precludes the determination of a single reference range for echocardiographic measurements that may be widely applied to all purebred and crossbred dogs.1,3,9 While normal echocardiographic parameters have been published for several other breeds, no such information is available for Border Collies.
The effect of breed on the echocardiogram is controversial. Athletic ability and susceptibility to heart disease are undoubtedly influenced by dog breed and body conformation, and it would be surprising if breed of dog did not also affect the size and function of the heart.3,4,9 A similar situation is recognized in horses, where racing breeds have proportionally larger hearts than stock and draft breeds.37 A less extreme situation is seen in man, where race and exercise status significantly affect the normal reference ranges of various echocardiographic measurements.38,39
When compared to the general population of dogs, Border Collies have a larger LVIDd, a finding consistent with previously reported echocardio- graphic measurements in other athletic breeds including Whippets,40 Greyhounds,4 and Alaskan Sled Dogs.41 This is reasonable since these are athletic breeds, and heart size is a key determinant of cardiac output and, hence, aerobic capacity and exercise performance.42-44 Although the increase in LVIDd may be the result of athletic training rather than a breed effect, this is unlikely here, since none of the Border Collies were reported to be actively training for athletic competition. It is also unlikely that the larger LVIDd in Border Collies was due to slower heart rate, as no correlation between heart rate and chamber size was found.
Table 4 Adjusted means comparison between Border Collies (BC) and a general dog population.
LV end-systolic dimension is determined by the degree of myofiber shortening, which, in turn, is affected by the inotropic state of the myocardium and by afterload. The increased LVIDs in healthy Border Collies is likely to reflect the fact that with the larger diastolic chamber dimension in this breed, minimal myocyte shortening is required to generate an adequate resting stroke volume (stroke volume = end diastolic volume - end systolic volume).45 Furthermore, the large LVIDs is conducive to significant increases in stroke volume and cardiac output during exercise in response to exercise-induced sympathetic stimulation.
In one previous canine study, 95% of normal dogs had a FS >25%; of the dogs with FS <25%, Greyhounds were disproportionately represented.46 Greyhounds and Alaskan Sled Dogs are bred for their athletic ability and appear to have larger, heavier hearts and unusually low values for FS even after prolonged periods of time not exercising.4,41 Data in the current study of healthy Border Collies confirmed that, like other athletic breeds, healthy Border Collies had a relatively low FS. The Border Collies had significantly lower FS than the general population of dogs used for comparison in this study. The FS of Border Collies is also lower than those previously reported in several other studies providing nonbreed specific M-mode normals.3,47-49
Fractional shortening is an index of systolic function calculated from the LVIDd and LVIDs. The normal value of this index assumes normal left ventricular morphology, afterload, preload and con- tractility.50-55 Therefore, while it is possible that the low FS observed in the Border Collies in this study resulted from an abnormal inotropic state, reduced preload, or increased afterload, these abnormalities are unlikely based on normal history, physical examination, laboratory screening, and arterial blood pressure measurements as well as normal 2D and color Doppler echocardiographic findings in each dog. Furthermore, LV sphericity index, LVPEP/LVET, and LV MPI measurements obtained from the Border Collies were normal based on published values, arguing against reduced inotropic state as cause of the low FS values.22,29
The IVSd was significantly less in the Border Collie group compared to the mixed population of dogs. Similar differences in septal thickness have been noted in peopleofvarying ethnicity, with normal men and women of black African descent having greater septal thickness than those of white European descent.38 The consequence of this difference with regard to athletic performance is unclear; however, the difference has clinical implications related to institution of antihypertensive treatment. Similarly, the difference in septal thickness between Border Collies and the general population of dogs has clinical implications related to the initiation of reverse remodeling treatments for myocardial disease.
Limitations of this study include the possibility that the Border Collies, although normal based on history, physical examination, 2D echocardio- graphic examination, and color Doppler may have had mild, subclinical heart disease. This possibility cannot be absolutely refuted without repeated follow up examinations. This same limitation applies to all previous studies defining normal reference ranges that were not longitudinal studies. However, the presence of normal systolic time intervals, normal LV myocardial performance index, and normal sphericity index support breed difference rather than subclinical myocardial disease as the reason for larger LV dimensions and lower FS in Border Collies. Similarly, the presence of valvular disease is unlikely, given the lack of structural valvular lesions and the fact that normal healthy dogs may have trace to physiologically mild mitral, tricuspid, and pulmonic regurgitations.
Conclusions
It is important that clinicians are aware of the differences in some echocardiographic parameters in healthy Border Collies compared to the general dog population. Failure to recognize these differences may lead to a misdiagnosis of myocardial failure. The values reported in this study should be used as the breed specific reference values for Border Collies.
Conflict of interest
None.
References
- Jacobs GJ, Mahjoob K. Multiple regression analysis, using body size and cardiac cycle length, in predicting echocardiographic variables in dogs. Am J Vet Res 1988;49:1290-1294.
- Sisson D, Schaeffer D. Changes in linear dimensions of the heart, relative to body weight, as measured by M-mode echocardiography in growing dogs. Am J Vet Res 1991;52:1591-1596.
- Morrison SA, Moise NS, Scarlett J, Mohammed H, Yeager AE. Effect of breed and body weight on echocardiographic values in four breeds of dogs of differing somatotype. J Vet Intern Med 1992;6:220-224.
- Snyder PS, Sato T, Atkins CE. A comparison of echocardiographic indices of the non-racing healthy greyhound to reference values from other breeds. Vet Radiol Ultrasound 1995;36:387-392.
- Bayon A, Fernandez del Palacio MJ, Montes AM, Gurierrez Panizo C. M-mode echocardiography study in growing Spanish mastiffs. J Small Anim Pract 1994;35:473-479.
- Herrtage M. Echocardiographic measurements in the normal boxer. In: ProcEurSoc Vet Intern Med 4th Ann Cong 1994. p. 172.
- Crippa L, Ferro E, Melloni E, Brambilla P, Cavalletti E. Echocardiographic parameters and indices in the normal beagle dog. Lab Anim 1992;26:190-195.
- Haggstrom J, Hamlin RL, Hanson K, Kvart C. Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. J Small Anim Pract 1996;37:69-75.
- Gooding JP, Robinson WF, Mews GC. Echocardiographic assessment of left ventricular dimensions in clinically normal English Cocker Spaniels. Am J Vet Res 1986;47:296-300.
- Page A, Edmunds G, Atwell RB. Echocardiographic values in the greyhound. Aust Vet J 1993;70:361-364.
- Calvert CA, Brown J. Use of M-mode echocardiography in the diagnosis of congestive cardiomyopathy in Doberman pinschers. J Am Vet Med Assoc 1986;189:293-297.
- Koch J, Pederson HD, Jensen AL, Flagstad A. M-mode echocardiographic diagnosis of dilated cardiomyopathy in giant breed dogs. J Vet Med 1996;43:297-304.
- DeMadron E. Update on normal M-mode echocardiographic values in the dog. PractMedChirAnim Comp 1995;30:647-657.
- Della Torre P, Kirby A, Church D, Malik R. Echocardiographic measurements in greyhounds, whippets, and Italian greyhounds - dogs with similar conformation but different size. Aust Vet J 2000;78:49-55.
- Lonsdale R, Labuc R, Robertson I. Echocardiographic parameters in training compared with non-training greyhounds. Vet Radiol Ultrasound 1998;39:325-330.
- Goncalves AC, Orton EC, Boon JA, Salman MD. Linear, logarithmic, and polynomial models of M-mode echocardiographic measurements in dogs. Am J Vet Res 2002;63:994-999.
- Rishniw M, Erb HN. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429-435.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072-1083.
- O'Rourke RA, Hanrath P, Henry WN, Hugenholtz PG, Pisa Z, Roelandt J, Tanaka M. Report of the joint International Society and Federation of Cardiology/World Health Organization task force on recommendations for standardization of measurements from m-mode echocardiograms. Circulation 1984;69:854A-857A.
- O'Grady M, Bonagura J, Powers J, Herring DS. Quantitative cross sectional echocardiography in the normal dog. Vet Radiol Ultrasound 1986;27:34-49.
- Serres F, Chetboul V, Gouni V, Tissier R, Sampedrano CC, Pouchelon JL. Diagnostic value of echo-Doppler and tissue Doppler imaging in dogs with pulmonary arterial hypertension. J Vet Intern Med 2007;21:1280-1289.
- Dukes-McEwan J, Borgarelli M, Tidholm A, Vollmar AC, Haggstrom J. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J Vet Card 2003;5:7-19.
- Kirberger R, Bland van den Berg P, Grimbeek R. Doppler echocardiography in the normal dog: part II factors affecting blood flow and a comparison between left and right heart blood flow. Vet Radiol Ultrasound 1992;33:380-386.
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167-184.
- Brown D, Knight D, King R. Use of pulsed wave Doppler echocardiography to determine aortic and pulmonary velocity and flow variables in clinically normal dogs. Am J Vet Res 1991;52:543-550.
- Boon JA. The two-dimensional echocardiographic exam. In: Veterinary echocardiography. 2nd ed. Ames: Blackwell Publishing; 2011. p. 71-72.
- Kirberger R, Bland-van den Berg P, Darazs B. Doppler echocardiography in the normal dog: part I, velocity findings and flow patterns. Vet Radiol Ultrasound 1992;33:370-379.
- Yuill CD, O'Grady MR. Doppler-derived velocity of blood flow across the cardiac valves in the normal dog. Can J Vet Res 1991;55:185-192.
- Teshima K, Asano K, Iwanaga K, Koie H, Uechi M, Kato Y, Kutara K, Kanno N, Seki M, Edamura K, Hasegawa A, Tanaka S. Evaluation of left ventricular Tei index (index of myocardial performance) in healthy dogs and in dogs with mitral regurgitation. J Vet Med Sci 2007;69:117-123.
- Zoghbi WA, Eriquez-SaranoM, FosterE, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802.
- Uehara Y, Takahashi M. Quantitative evaluation of the severity of mitral insufficiency in dogs by color flow Doppler method. J Vet Med Sci 1996;58:249-253.
- Muzzi RA, de Araujo RB, Muzzi LA, Pena JL, Silva EF. Regurgitant jet area by Doppler color flow mapping: quantitative of mitral regurgitation severity in dogs. J Vet Card 2003;5:33-38.
- Schmidt A, Oswaldo C, Almeida F, Pazin A, Marin-Neto JA, Maciel BC. Valvular regurgitation by color Doppler echocardiography. Arq Bras Cardiol 2000;74:273-281.
- Choong C, Abascal V, Weyman J, Levine RA, Gentile F, Thomas JD, Weyman AE. Prevalence of valvular regurgitation by Doppler echocardiography in patients with structurally normal hearts by two dimensional echocardiography. Am Heart J 1989;117:636-642.
- Sahn DJ, Maciel BC. Physiological valvular regurgitation and the potential for iatrogenic heart disease. Circulation 1988; 78:1075-1077.
- Yoshida K, Yoshikawa J, Shakudo M, Akasaka T, Jyo Y, Takao S, Shiratori K, Koizumi K, Okumachi F, Kato H. Color Doppler evaluation of valvular regurgitation in normal subjects. Circulation 1988;78:840-847.
- Kline H, Foreman JH. Heart and spleen weights as a function of breed and somatotype. Equine Exerc Physiol 1991;3: 17-21.
- Chaturvedi N, Athanassopoulos G, McKeigue PM, Marmot MG, Nihoyannopoulos P. Echocardiographic measures of left ventricular structure and their relation with rest and ambulatory blood pressure in blacks and whites in the United Kingdom. J Am Coll Cardiol 1994;24:1499-1505.
- Pelliccia A, Maron MS, Naron BJ. Assessment of left ventricular hypertrophy in a trained athlete: differential diagnosis of physiologic athlete's heart from pathologic hypertrophy. Prog Cardiovasc Dis 2012;54:387-396.
- Bavegems VC, Duchateau L, Polis IE, van Ham LM, de Rick AF, Sys SU. Detection of innocent systolic murmurs by auscultation and their relation to hematologic and echo- cardiographic findings in clinically normal Whippets. J Am Vet Med Assoc 2011;238:468-471.
- Stepien RL, Hinchcliff KW, Constable PD, Olson J. Effect of endurance training on cardiac morphology in Alaskan sled dogs. J Appl Physiol 1998;85:1368-1375.
- Colan SD. Mechanics of left ventricular systolic and diastolic function in physiologic hypertrophy of the athlete heart. Cardiol Clin 1992;10:227-240.
- Young LE. Cardiac responses to training in 2-year-old thoroughbreds: an echocardiographic study. Equine Vet J Suppl 1999;30:195-198.
- Stone HL. Cardiac function and exercise training in conscious dogs. J Appl Physiol 1977;42:824-832.
- Yang SS, Bentivoglio LG, Maranhao V, Goldberg H. Cardiac volume. In: From cardiac catheterization data to hemodynamic parameters. 2nd ed. Philadelphia: FA Davis Company; 1978. p. 101-151.
- Cornell CC, Kittleson MD, Della Torre P, Haggstrom J, Lombard CW, Pedersen HD, Vollmar A, Wey A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311-321.
- Lombard C. Normal values of the canine M-mode echocardiogram. Am J Vet Res 1984;45:1591-1598.
- Boon J, Wingfield WE, Miller CW. Echocardiographic indices in the normal dog. Vet Rad 1983;24:214-221.
- Luis Fuentes V. Current issues in echocardiography. In: Proc 18th ACVIM Forum, Seattle, WA 2000. p. 88-92.
- Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol 1983;45:169-189.
- Fagard RH. Athlete's heart: a meta-analysis of the echocardiographic experience. Int J Sports Med 1996;17: S140-S144.
- Fagard RH. Effect of training on left ventricular structure and functioning of the normotensive and the hypertensive subject. Blood Press Monit 1997;2:241-245.
- Kittleson M. Left ventricular function and failure - part I. Comp Cont Educ Pract 1994;16:287-308.
- Kittleson M. Left ventricular function and failure - part II. Comp Cont Educ Pract 1994;16:1001-1017.
- Aurigemma G, Douglas P, Gaasch W. Quantitative evaluation of left ventricular structure, wall stress, and systolic function. In: Otto C, editor. The practice of clinical echocardiography. 2nd ed. Philadelphia: WB Saunders; 2002. p. 65-87.
^Наверх









