Alternative methods for the measurement of the minimal ductal diameter of a patent ductus arteriosus in a dog

Author information
Sanders R.A., Olivier N.B. Alternative methods for the measurement of the minimal ductal diameter of a patent ductus arteriosus in a dog // J Vet Cardiol. 2016 Dec;18(4):372-376.
Abstract
A two and half-year-old, 24 kg, spayed female German Shepherd was presented for evaluation of a suspected patent ductusarteriosus (PDA). Transthoracic echocardiographic examination confirmed a left to right shunting PDA. Closure of the PDA was recommended, and the owners elected to have minimally invasive transcatheter closure of the patent ductus arteriosus (PDA) performed. Standard ductalangiography failed to provide adequate measurements of the minimal ductal diameter (MDD). Alternative methods of measuring the MDD using an angiography catheter and a balloon catheter were performed. The patent ductusarteriosus (PDA) was occluded using an Amplatz® Canine DuctalOccluder without complication. Further evaluation of these techniques is needed to determine the accuracy, overall clinical efficacy, and safety of using alternative methods for the measurement of the MDD of a PDA.
KEYWORDS: Canine; Congenital; Interventional
Abbreviations
- ACDO Amplatz® canine duct occluder
- ASD atrial septal defect
- MDD minimal ductal diameter
- PDA patent ductus arteriosus
A two and halfeyear-old, 24 kg, spayed female German Shepherd was presented to the cardiology service at Michigan State University Veterinary Medical Center for evaluation of a suspected patent ductus arteriosus (PDA). Upon presentation, a grade IV/VI left basilar continuous murmur was heard and femoral pulses were found to be bounding. A transthoracic echocardiogram was performed confirming the presence of a left to right shunting PDA. The echocardiogram also revealed mild dilation of the left atrium and ventricle, mild mitral regurgitation, and continuous turbulence in the main pulmonary artery. The minimal ductal diameter (MDD) could not be measured, and the shape of the PDA could not be identified by transthoracic echocardiographic * Corresponding author. examination in this patient. Closure of the patent ductus arteriosus (PDA) was recommended, and the owners elected to have minimally invasive transcatheter closure of the PDA performed.
The dog was anesthetized for transcatheter closure of the PDA using a standard anesthetic protocol. The patient was premedicated with 0.5 mg/kg (12 mg) of methadone3 IM, induced with 3.9 mg/kg (93 mg) of propofolb IV, and maintained under general anesthesia using isofluranec to effect in 100% oxygen. The patient also received lactated Ringer’s solutiond fluids at 10 mL/kg/hr throughout the procedure and 20 mg/kg (480 mg) of cefazoline IV q 90 min perioperatively. Once anesthetized, a 7 Fr introducer sheathf was placed in the femoral artery using the modified Seldinger technique and a 7 Fr quadripolar electrophysiology catheterg was advanced transorally through the esophagus to the level of the heart under fluoroscopic guidance to be used as a marker catheter to calibrate the fluoroscopic measurement system. At the time, this procedure was performed the pressure injector malfunctioned, and as such, hand injections were required to perform ductal angiography. A 5 Fr pediatric pigtail catheterh was advanced to the level of the ascending aorta, and an angiographic study was performed. Accurate measurement of the minimal ductal diameter (MDD) could not be obtained from this angiographic study due to the high flow rate through the patent ductusarteriosus (PDA) in this large patient. In an attempt to gain an accurate measurement, a 7 Fr reverse Berman angiography catheter* i * was then advanced to be within the ampulla of the PDA. The balloon of the angiography catheter was inflated within the ampulla of the PDA in an effort to reduce or occlude flow through the PDA, and an angiographic study was performed.
a Methadone-Bioniche Parma USA LLC, Lake Forest, IL, USA.
b Propofol-Abbott Laboratories, North Chicago, IL, USA.
c Isoflurane-Abbott Laboratories, North Chicago, IL, USA.
d Lactated Ringer’s Solution (LRS)-Hospira, Inc, Lake Forest, IL, USA.
e Cefazolin-Hospira, Inc, Lake Forest, IL, USA.
f 7 Fr AVANTI®+, Cordis, Hialeah, FL, USA.
g 7 Fr Decapolar electrophysiologic catheter, St. Jude Medical St. Paul, MN, USA.
h 5 Fr Pediatric Pigtail diagnostic catheter, Cook, Bloomington, IN, USA.
i 7 Fr Reverse Berman Angiography Catheter, Arrow, Morris- ville, NC, USA.
The MDD was measured to be 4.2 ± 0.5 mm (Fig. 1), but there was a concern that this was not an accurate measurement due to possible distortion of the size of the patent ductusarteriosus (PDA) caused by the inflation of the balloon or possible distortion of the minimal ductal diameter (MDD) caused by reduction or occlusion of shunt flow. Following this, a 6 Fr MPA catheterj was used to pass a 0.035 in guidek wire through the PDA into the main pulmonary artery. A 9 x 4 mm balloon dilation (nominal pressure = 8.9 and burst pressure = 9.8) catheterl was advanced over the guide wire and positioned across the minimal ductal diameter (MDD). The balloon was gently inflated with a dilute contrast solution by hand. Care was taken to maintain a low inflation pressure within the balloon in an attempt to avoid stretching or tearing the MDD of the PDA. The MDD diameter of the patent ductusarteriosus (PDA) created a 5.9 ± 0.5 mm narrowing in the balloon (Fig. 2). Based on this measurement, a 10 mm Amplatz® Canine Ductal Occluderm (ACDO) was selected to have an ACDO waist-to- angiographic measurement of the balloon waist ratio of 1.7. The ACDO was placed in a standard fashion as previously described [1]. Following placement of the ACDO, angiography confirmed appropriate positioning of the Amplatz® Canine Ductal Occluderm (ACDO) and complete closure of the PDA. Recovery from anesthesia was uneventful. An echocardiogram was performed 24 h after ductal occlusion and revealed no residual flow through the patent ductusarteriosus (PDA).
Discussion
Multiple morphologies of PDAs have been identified in veterinary [2] patients, and multiple devices have been used to successfully occlude PDAs [1,3—5]. In both human and veterinary medicine, the choice of device used to occlude a PDA is based on echo- cardiographic and angiographic classification of the PDA and measurement of the minimal ductal diameter (MDD) of the PDA [6—9]. Dislodgement of the closure device is a recognized complication in both human and veterinary medicine and has been reported for all types of devices [1,3,9,10]. Device dislodgement is rare in humans and is thought to occur due to operator error or error of measurement of the MDD resulting in an inappropriately sized device [8]. There has been a single perioperative dislodgement of an Amplatz® Canine Ductal Occluderm (ACDO) described in veterinary medicine [1]. The patient was the second clinical patient to have an ACDO placed, and the authors hypothesized that the Amplatz® Canine Ductal Occluderm (ACDO) waist-to- angiographic minimal ductal diameter (MDD) ratio was to low for secure placement of the device.
j 6 Fr MPA diagnostic catheter, Cook, Bloomington, IN, USA. k 0.035 inch 150 cm Wire Guide, Cook, Bloomington, IN, USA.
l 9 mm Advance® ATB PTA Dilatation Catheter, Cook, Bloomington, IN, USA.
m 10 mm Amplatz® canine duct occluder, St. Jude Medical St. Paul, MN, USA.
Figure 1 Right lateral angiogram using a reverse Berman angiography catheter with the balloon inflated
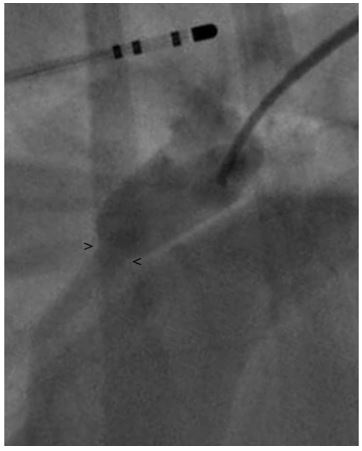
. The minimal ductal diameter (MDD) diameter is indistinct and difficult to accurately measure. Note the electrophysiology catheter within the esophagus used a marker catheter. > and < mark the points measurement of the MDD was made. MDD, minimal ductal diameter.
In a report detailing delayed embolization of an ACDO in a dog, in addition to unsupervised activity, inaccurate assessment of morphology and estimation of ductal dimensions were considered as a possible reason for the delayed device embolization [11]. As such, it is clear that accurate measurement of the MDD is critical to optimal device choice and secure device placement. In humans, an alternative fluoroscopic view has been described to enhance the visualization of the MDD of the patent ductusarteriosus (PDA) [12]. Accurate sizing of any type of lesion is essential to determine the appropriate sized closure device. When performing transcatheter closure of an atrial septal defect (ASD), a compliant sizing balloon catheter is placed across the lesion and inflated with dilute contrast until a waist in the balloon is visible on fluoroscopic imaging to obtain the diameter of the lesion [13].An alternative method using echocardiographic evaluation of flow through the ASD while inflating a sizing balloon catheter to determine when the balloon occluded flow through the ASD has been described and is thought to result in less overstretching of the defect [14]. Recently, a technique using a sizing balloon catheter to enhance the accuracy of the assessment of the MDD in a patent ductusarteriosus (PDA) has been described in people and was performed successfully in four people without complication [15].
Figure 2 Right lateral fluoroscopic image demonstrating a balloon dilation catheter inflated across the PDA resulting in a waist at the MDD
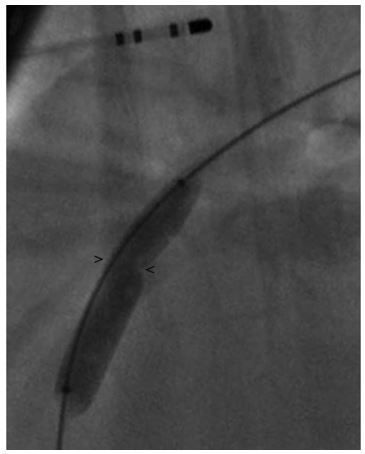
> and < mark the points measurement of the minimal ductal diameter (MDD) was made. MDD, minimal ductal diameter; PDA, patent ductus arteriosus.
This case report describes the first reported use of a similar technique in a dog and the use of a reverse Berman angiography catheter to provide an alternative method of obtaining a measurement of the minimal ductal diameter (MDD) of a PDA. We used a reverse Berman angiography catheter placed within the ampulla of the PDA with the balloon inflated to restrict flow through the PDA. While this resulted in an acceptable image to allow measurement of the patent ductusarteriosus (PDA), we were concerned that the measurement may have been artificially low (as the PDA may have appeared smaller with reduced flow through the ductus caused by inflation of the balloon). The measurement of the minimal ductal diameter (MDD) using the angiography catheter was much lower than the measurement using the balloon catheter (4.2 vs. 5.9, respectively). If the measurement obtained from the angiogram using the angiography catheter was used to size the Amplatz® Canine Ductal Occluderm (ACDO), then a 7—8 mm device would have been chosen. If this measurement is an underestimated, then an undersized device may have been placed and could have resulted in an increased risk of device embolization. However, it is not known if the measurement obtained using the reverse Berman catheter or the measurement obtained using the balloon dilation catheter is more accurate. Further evaluation of which technique will provide the most accurate measurement is needed.
In this patient, a balloon dilation catheter was used to aid in the visualization and measurement of MDD of the patent ductus arteriosus (PDA). While we believe a sizing balloon catheter to be a more appropriate catheter to measure the MDD, one was not available at the time of the procedure. A sizing balloon catheter is a very compliant balloon compared to a standard balloon dilation catheter and may be less likely to distort the MDD. It may be that the stiffness of the balloon dilation catheter may have resulted in distortion of the MDD in this patient. Use of a sizing balloon catheter to aid in the measurement of the MDD may allow for more accurate measurement of the firm ductal walls and may help to reduce the risk of device embolization. We recommend inflating the balloon just until flow through the patent ductusarteriosus (PDA) has been occluded to minimize the risk of tearing or causing significant stretching of the balloon similar to the technique described for use in human patients with ASD [14]. The use of a sizing balloon catheter retrograde may also be useful to measure the MDD in patients that are too small to allow easy catheterization of the femoral artery. An additional benefit of this technique could include a reduction in the total dose of contrast material given to a patient.
In conclusion, this case report details the first reported case of alternative methods to measure the minimal ductal diameter (MDD) of a PDA in veterinary patients. No complications were noted. Further evaluation of these techniques is needed to determine the accuracy, overall clinical efficacy, and safety of using these alternative methods for the measurement of the MDD of a patent ductus arteriosus (PDA).
Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
References
- Nguyenba TP, Tobias AH. The Amplatz® canine duct occluder: a novel device for patent ductus arteriosus occlusion. J Vet Cardiol 2007;9:109-17.
- Miller MW, Gordon SG, Saunders AB, Arsenault WG, Meurs KM, Lehmkuhl LB, Bonagura JD, Fox PR. Angiographic classification of patent ductus arteriosus morphology in the dog. J Vet Cardiol 2006;8:109-14.
- Singh MK, Kittleson MD, Kass PH, Griffiths LG. Occlusion devices and approaches in canine patent ductus arteriosus: comparison of outcomes. J Vet Intern Med 2012;26:86-92.
- Hogan DF, Green HW, Sanders RA. Transcatheter closure of patent ductus arteriosus in a dog with a peripheral vascular occlusion device. J Vet Cardiol 2006;8:139-43.
- Schneider M, Hildebrandt N, Schweigl T, Schneider I, Hagel KH, Neu H. Transvenous embolization of small patent ductus arteriosus with single detachable coils in dogs. J Vet Intern Med 2001;15:222-8.
- Grifka RG. Transcatheter closure of the patent ductus arteriosus. Catheter Cardiovasc Interv 2004;61:554-70.
- Glaus TM, Martin M, Boller M, Stafford Johnson M, Kutter A, Fluckiger M, Tofeig M. Catheter closure of patent ductus arteriosus in dogs: variation in ductal size requires different techniques. J Vet Cardiol 2003;5:7-12.
- Baruteau AE, Hascoet S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, Belli E, Petit J, Pass R. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis 2014;107:122-32.
- Brunetti MA, Ringel R, Owada C, Coulson J, Jennings JM, Hoyer MH, Everett AD. Percutaneous closure of patent ductus arteriosus: a multiinstitutional registry comparing multiple devices. Catheter Cardiovasc Interv 2010;76: 696-702.
- Sultan M, Ullah M, Sadiq N, Akhtar K, Akbar H. Transcatheter device closure of patent ductus arteriosus. J Coll Physicians Surg Pak 2014;24:710-3.
- Carlson JA, Achen SA, Saunders AB, Gordon SG, Miller MW. Delayed embolization of an Amplatz(®) canine duct occluder in a dog. J Vet Cardiol 2013;15:271-6.
- Garg N, Moorthy N. An alternative angiographic view to unmask the hidden patent ductus arteriosus during device closure. Catheter Cardiovasc Interv 2012;80:937-9.
- Harper RW, Mottram PM, McGaw DJ. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv 2002;57:508-24.
- Carlson KM, Justino H, O'Brien RE, Dimas VV, Leonard GT, Pignatelli RH, Mullins CE, Smith EO, Grifka RG. Transcatheter atrial septal defect closure: modified balloon sizing technique to avoid overstretching the defect and oversizing the Amplatzer septal occluder. Cathet Car- diovasc Interv 2005;66:390-6.
- Butera G, Lovin N, Basile DP. Patent ductus arteriosus balloon sizing: a new technique to evaluate the size in complex cases. Catheter Cardiovasc Interv 2016;87:1135-7.
^Наверх









